option a materials Notes
Materials science introduction
Classifying materials
- Materials science involves understanding the structure and properties of a material and matching these to suitable applications.
- Type of bonding is one classification system employed by materials science, and four common types of material are metallics, ceramics, polymers, and composites. Each type is suitable for different end uses.
- Metallic substances exhibit metallic bonding. This makes them strong, malleable, and good conductors of heat and electricity. Approximately two-thirds of all elements are metals. The development of alloys has designed metallic materials suitable for many applications.
- At the atomic level, the freely moving electrons in metallic bonding confer ductility and strength, as well as conductivity.
- Ceramics are traditionally inorganic non-metallic solids formed between metals and non- metals. They have a crystalline structure and their ionic bonding means they are brittle and usually show insulating properties, such as the ceramics familiar in plates and cups.

- However, a wide variety of ways of combining metals and non-metals leads to many ceramics with various uses. A compound of thallium, barium, calcium, copper, and oxygen forms a superconductor whereas glass is an amorphous insulating ceramic material.
- Polymers (also known as plastics) form a third classification of materials based on bonding. Plastics are covalently bonded long chain molecules. There are many uses and types of plastics and the industry is growing rapidly.
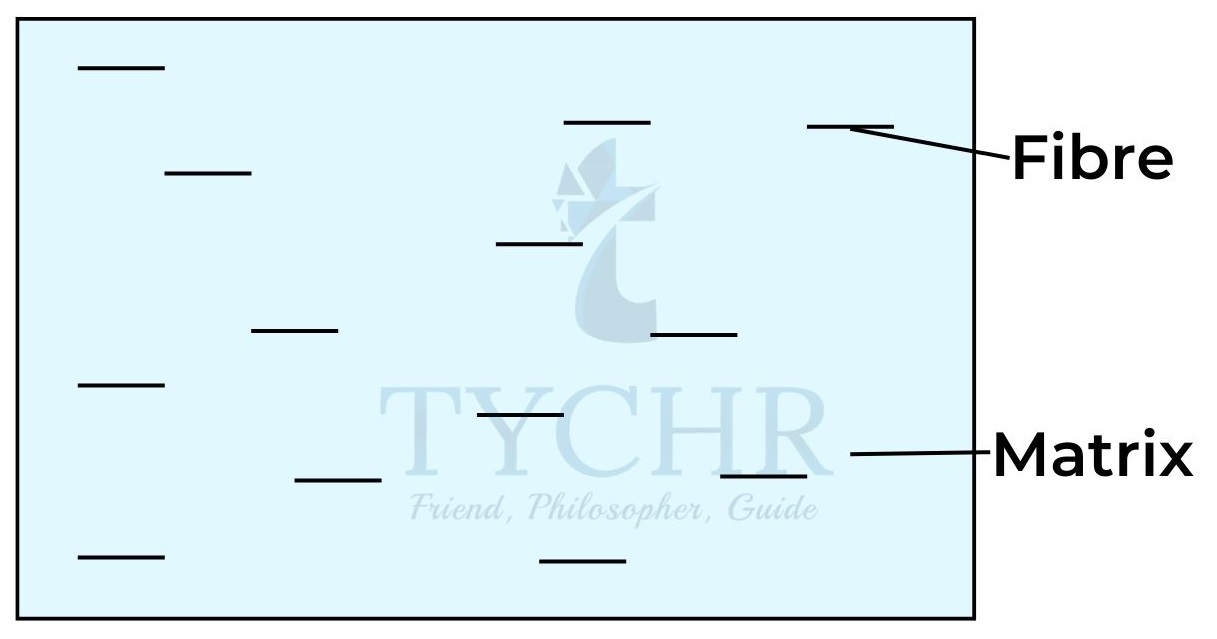
- Composites are mixtures composed of two distinct phases: a reinforcing phase embedded in a matrix. Each substance retains its own properties; however the composite has specific properties not shown by either part of the mixture individually.
- Straw and clay formed an early composite used to build huts.
Bond triangle diagrams
- Bonds between metals and non-metals vary from ionic to covalent in relation to the electronegativity difference between the two types of atom.
- The greater the electronegativity difference, the higher the ionic character.
- A material with polar covalent bonds exhibits some ionic and some covalent character.
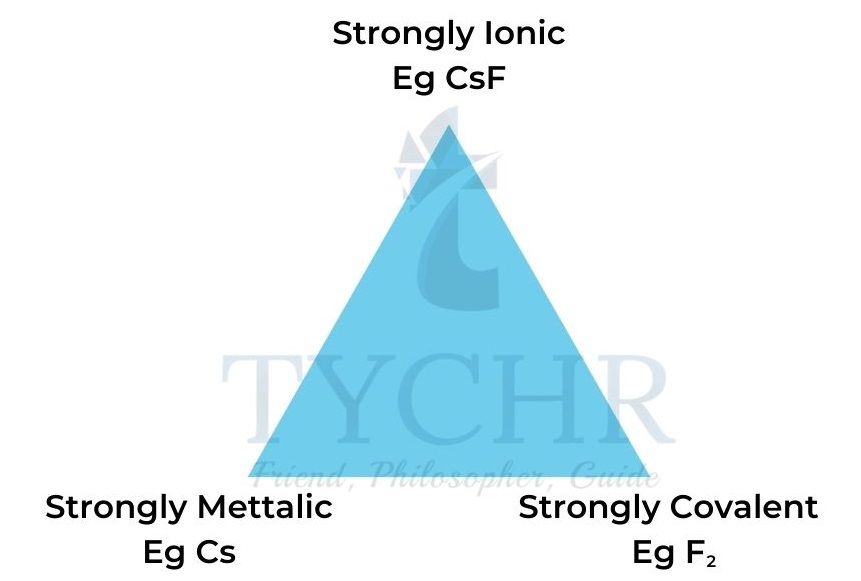
- The bonding in a material is determined by the magnitude and difference of the electronegativities (χ) of the constituent elements. This is illustrated by the triangle of bonding shown in Figure 2.
Metals and inductively coupled plasma (ICP) spectroscopy
Reduction of metals
- Some metals such as gold can be mined directly as the element. However, most metals exist in nature in their oxidized states in compounds; for example, aluminium is found in bauxite as aluminium oxide, Al2O3.
- These metals can be extracted from their ores and are then often alloyed to give them useful properties.
Reduction of iron ore in the blast furnace
- Reduction is carried out on a large scale industrially to obtain iron from iron ore. Most of the iron extracted is then processed further to produce steel. Iron ore is mainly the oxides Fe2O3 and Fe3O4 which are reduced by carbon in the form of coke in a blast furnace.
- Coke is heated to form carbon dioxide, which reacts with more coke to form carbon monoxide in the reducing furnace:
C(s) + O2(g) → CO2(g) CO2(g) + C(s) → 2CO(g)
- Carbon monoxide is a good reducing agent (it is easily oxidized) and reacts with the iron ore to produce molten iron, which is collected from the furnace:
Fe2O3(s) + 3CO(g) → 2Fe(l) + 3CO2(g)
- At the very high temperatures in the blast furnace the coke can react directly with the iron ore and also act as a reducing agent itself:
Fe2O3(s) + 3C(s) → 2Fe(l) + 3CO(g)
- The carbon monoxide produced in this reaction can reduce more ore.
Reduction by a more reactive metal
- A second means of obtaining elemental metals is reduction by a more active metal. Pure copper can be obtained from aqueous copper(II) sulphate by a single replacement reaction with solid zinc, for example:
Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
- Other redox reactions can be used to reduce the oxidized metal. For example, passing hydrogen gas over heated copper(II) oxide reduces the copper(II) oxide to elemental copper, while the hydrogen is oxidized to the +1 state:
CuO(s) + H2(g) → Cu(s) + H2O(g)
Alloys
- Alloys are homogeneous mixtures of metals with other metals or non-metals.
- By taking a readily available metal and adding small amounts of another material to it, certain desired properties can be greatly enhanced. For example, steel is stronger than iron, and stainless steel is produced in many grades of different composition depending on the purpose.
- Copper alloys such as bronze and brass have increased resistance to corrosion compared with pure copper.
Paramagnetic and diamagnetic materials
- One property of interest in a metal or alloy is its response to a magnetic field.
- Paramagnetic materials are attracted to a magnetic field whereas diamagnetic materials create a magnetic field opposed to the applied field; and are therefore weakly repelled by an external magnetic field.
- In the atoms of a diamagnetic material the electrons are spin paired; for example, neon is diamagnetic with electron configuration 1s22s22p6
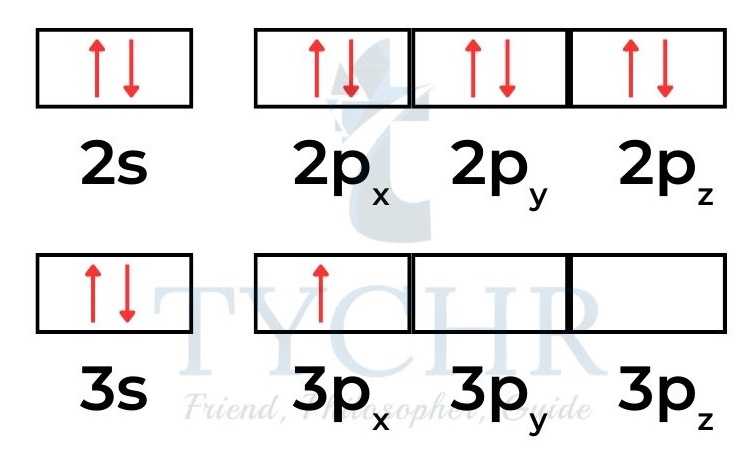
- so all 10 electrons exist in a paired state. Aluminium atoms, electron configuration 1s22s22p63s23p1 have one unpaired p electron that is capable of being attracted to an external electric field. Aluminium is paramagnetic:
- A spinning electron creates a magnetic dipole. The spins of unpaired electrons can be temporarily aligned in an external field, causing the material to be attracted to the applied magnetism. This is what happens in paramagnetic materials.
- In a ferromagnetic material the electron alignment induced by the magnetic eld can be retained, making a permanent magnet.
Spectroscopic methods
- Trace concentrations of elements such as heavy metals in water are difficult to determine by chemical tests but can be detected by spectrophotometry techniques. Qualitative analysis showing which metals are present can be carried out by exciting electrons to higher energy levels and detecting the characteristic wavelength of light emitted as these electrons return to lower energy levels; this is the process employed in atomic emission spectroscopy (AES) (figure 5). Concentrations (quantitative information) can be detected by the level of absorbance of this radiation in optical emission spectroscopy (OES).
Figure 5: As excited electrons return from an excited-state to a lower energy level they emit a characteristic wavelength of light
- In mass spectrometry (MS) ions are introduced into a mass spectrometer and separated according to their mass-to-charge ratio. The detector receives a signal proportional to the concentration of the ion reaching it thus allowing both identification (qualitative analysis) and quantification.
- Plasma can also be used for the atomization and/or excitation of samples for spectroscopy. Plasma is one of the four states of matter and it consists of free electrons, positive ions, and neutral atoms or molecules.
- Lightning, electric sparks, and the coloured neon lights used in advertising are all examples of matter in the plasma state.
Catalysts
Homogeneous and heterogeneous catalysts
- A catalyst increases the rate of a reaction and is left unchanged at the end of the reaction.
- At the conclusion of the reaction, a homogeneous catalyst is transformed into a product and occupies the same phase as the reactants.
- A heterogeneous catalyst is in a different phase than that of the reactants.
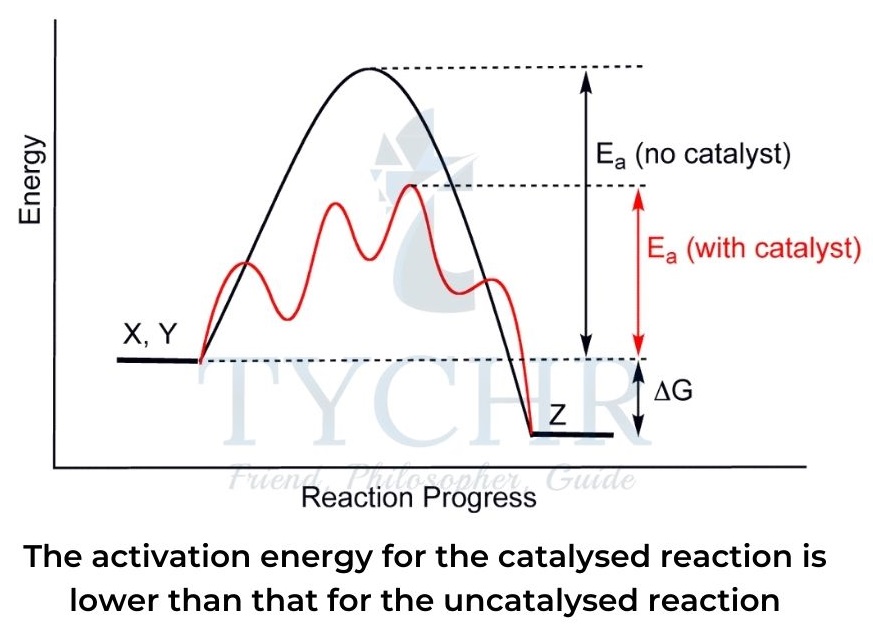
- Catalysts work by providing an alternative reaction pathway for the reaction that lowers the activation energy, as shown in a potential energy profile diagram in figure.
- A higher proportion of reactant particles therefore achieve the required activation energy as a result (see the Maxwell–Boltzmann distribution curve).
Mechanisms of catalysis
- The mechanism by which the activation energy is lowered varies between homogeneous and heterogeneous catalysts.
- A homogeneous catalyst forms bonds with one or more of the reactants resulting in either a reaction intermediate, which then further reacts, or an activated complex, which is a temporary transition state.
- In either case the energy needed for the reactant molecules to complete the reaction is reduced as the reaction occurs between reactant and catalyst rather than between one reactant and another.
- For example, in the general reaction:
A + B →AB
- A and B need to collide and overcome the activation energy for the reaction. In the catalysed reaction:
A + C → AC*
AC* + B → AB + C
- The collision between A and the catalyst C to form the intermediate AC* requires less energy than does the collision between A and B to form AB.
- In this way the homogeneous catalyst enters the reaction, but is left unchanged at the end.
- A heterogeneous catalyst is in a different phase from the reactants, usually a solid catalyst for a gaseous reaction or a reaction in solution. Transition metals are common heterogeneous catalysts.
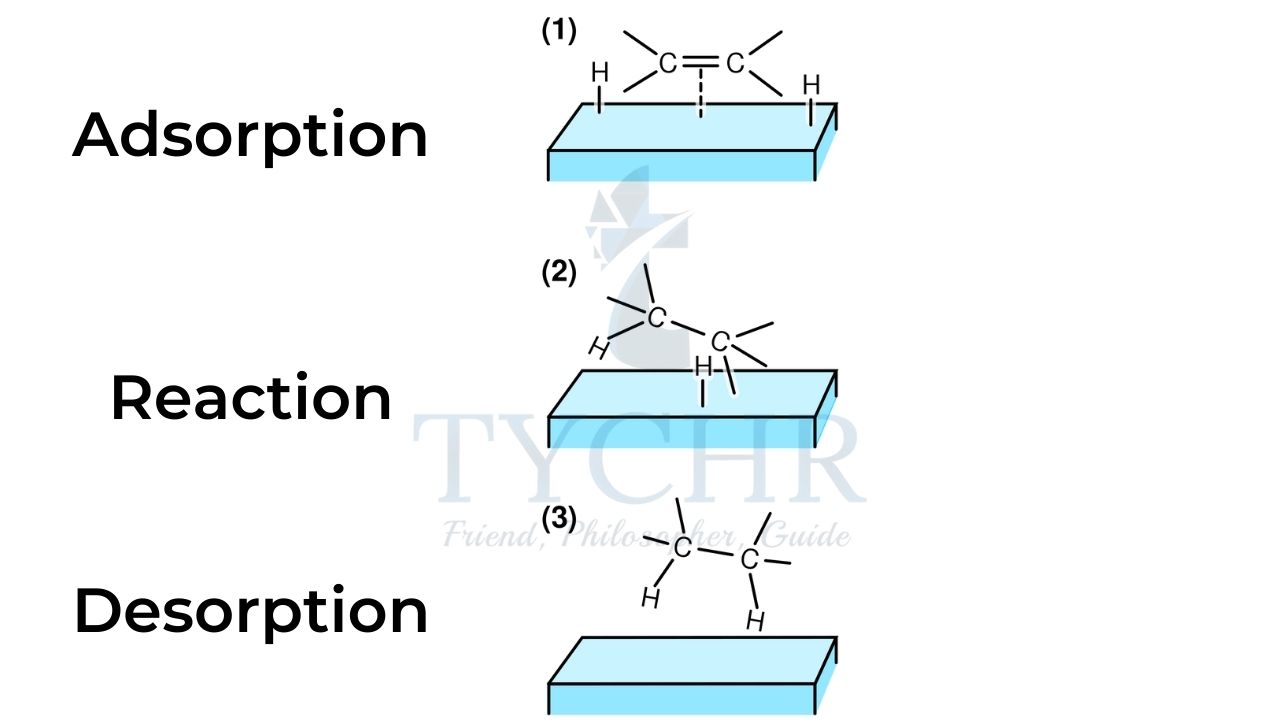
- Reactant molecules adsorb onto the heterogeneous catalyst at active sites.
- The reaction occurs on the surface of the catalyst, in one or several steps, and the products desorb from the surface of the catalyst.
Homogeneous and heterogeneous catalysts compared
Homogeneous catalysts | Heterogeneous catalysts |
high activity | high activity |
high selectivity | low selectivity |
difficult separation | simple separation |
low reaction temperature | high reaction temperature |
high adaptability | lower adaptability |
Table 1: Comparison between Homogeneous and heterogeneous catalysts
Nanocatalysts
- Nanoparticles are particles that have dimensions less than 100 nm and exhibit properties that differ from those of the bulk material. Individual molecules are usually not considered to be nanoparticles but small clusters of them may be.
- The use of nanoparticles has bridged the gap between homogeneous and heterogeneous catalysts. Due to their small size nanocatalysts are sometimes referred to as catalytic particles.
- Nanocatalysts generally have a high conversion efficiency because of their small size and large surface area. They can be engineered for maximum selectivity which reduces catalytic poisoning by unwanted substances.
- Nanocatalysts can also provide low energy consumption and a long lifetime.
- Many nanocatalysts contain various forms of carbon, including graphite, carbon nanotubes, fullerenes, and graphene.
Transition metal catalysts
- Ceramics provide some useful catalysts, but the most widely used inorganic catalysts are transition metals due to their variable oxidation states and high adsorption capacity.
- The variable oxidation states of transition metals allow them to enter many reactions as homogeneous catalysts.
- Transition metals also make good heterogeneous catalysts: many gases will adsorb to their surface.
- Some transition metals are toxic and should be used only if a suitable alternative is not available.
Zeolites
- Zeolites are microporous substances made of alumina silicate which has a cage-like structure providing a large surface area. Zeolites are cheap, plentiful, and occur naturally in many forms including pumice.

- A zeolite can act as a microscopic sieve, allowing only certain molecules through depending on their size and structure. Zeolites work by both adsorption and cation exchange.
- Zeolites are used to remove heavy metal ions from water supplies.
- Zeolites are also used as catalysts for cracking in the petroleum industry.
- Many washing powders contain zeolites to make washing in hard water locations more effective.
Liquid crystals
The properties of liquid crystals
- A state of matter that lies in between crystalline and liquid is known as liquid crystals. They are fluids whose physical properties are determined by the orientation of the molecules in relation to a specific material axis: Molecules in a liquid crystal can flow like liquids but line up in a crystalline order.
- Many liquid crystals have a linear or flat molecular structure with little branching. Long-chain alkyl groups that form long, thin, rigid molecules in the shape of rods or linear aromatic ring chains that form flat disc shapes are common in them.
- The liquid-crystal state is created by these chains’ capacity to align under a weak electric field.
- Liquid crystals are often polar molecules so they change orientation when an electric field is applied.
- To form useful liquid crystals a substance needs to be chemically stable and have a liquid- crystal phase that is stable over a suitable temperature range.
Transmitting light: Forming LCD displays
- Due to their low energy consumption, liquid-crystal displays are utilized in numerous lightweight applications like digital watches, calculators, and laptops.
- The orientation of the molecules in a liquid crystal determines whether or not they are able to transmit light. The display’s light and dark regions can be controlled by applying a small voltage to a thin film of the material.
- Pure liquid-crystal materials that exhibit liquid-crystal behaviour over a temperature range between the solid and liquid states are known as thermotropic liquid-crystal materials.
- Solutions that exhibit the liquid-crystal state at specific concentrations are known as lyotropic liquid crystals.
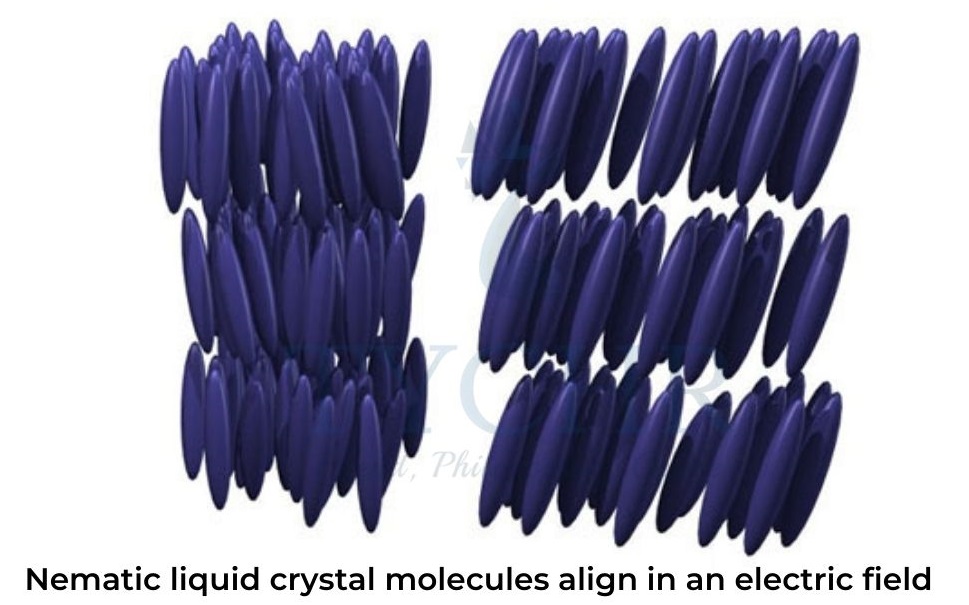
- The rod-shaped molecules that make up the nematic liquid-crystal phase are randomly distributed but typically align in the same direction.
- In an electric field the molecules of a nematic liquid crystal become oriented as shown in figure 4(b).
- Nematic liquid crystals are frequently arranged in layers that are perpendicular to one another in LCD displays, with each pixel being sandwiched between two polarized glass plates.
- Over a wide temperature range, the behaviour of thermotropic liquid crystals shifts.
- Biphenyl nitriles are thermotropic liquid crystals that naturally exist in the nematic phase as shown in figure 4(b), their rod-shaped molecules distributed randomly but on average pointing in the same direction. Increased thermal agitation disrupts this directional order until it is lost, as in figure 4(a), when the normal liquid phase is formed.
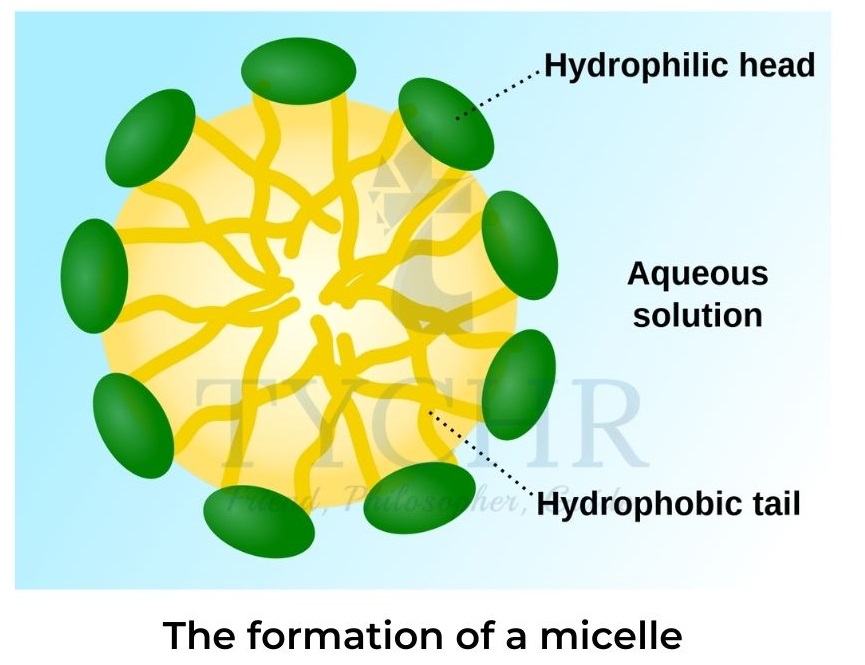
- Soap forms spherical micelles (figure 7).The polar ends on the outside of the sphere are surrounded by water molecules with non-polar oil or grease encapsulated in the centre.
- At high concentrations rod-like micelles are formed that have liquid-crystal properties and can form bilayer sheets.
Polymers
What are polymers?
- The word “polymer” means “many parts”.
- A polymer is a molecule i.e. formed by joining many small molecules called monomers.
- These are repeating monomer units,whose structures can be reformed in various ways, to give materials with desired properties.
- An addition reaction is the one in which two or more molecules join together to give a single product.
- Polyethene is an addition polymer made of ethene monomer units.
nCH2= CH2 [-CH2-CH2-]n
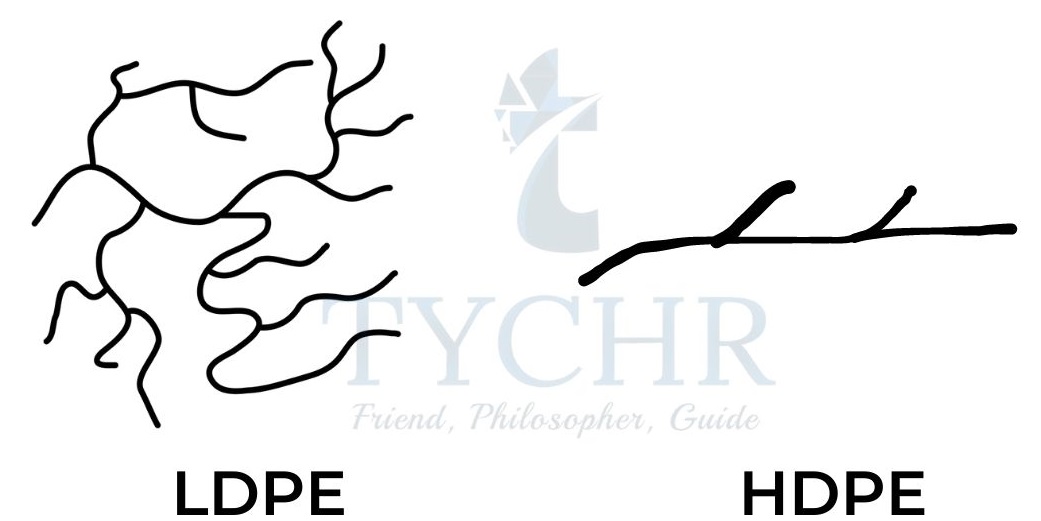
High density and Low density polyethene
Property | Low Density Polyethene (LDPE) | High Density Polyethene (HDPE) |
Chemical structure | More branching | Less branching, more linear |
Density | Low density | High density |
Flexibility | Low crystallinity, and therefore more flexible | High crystallinity, which makes it tougher and more rigid |
Strength | Low tensile strength | Greater tensile strength |
Table 2: Comparison between High density and Low density polyethene
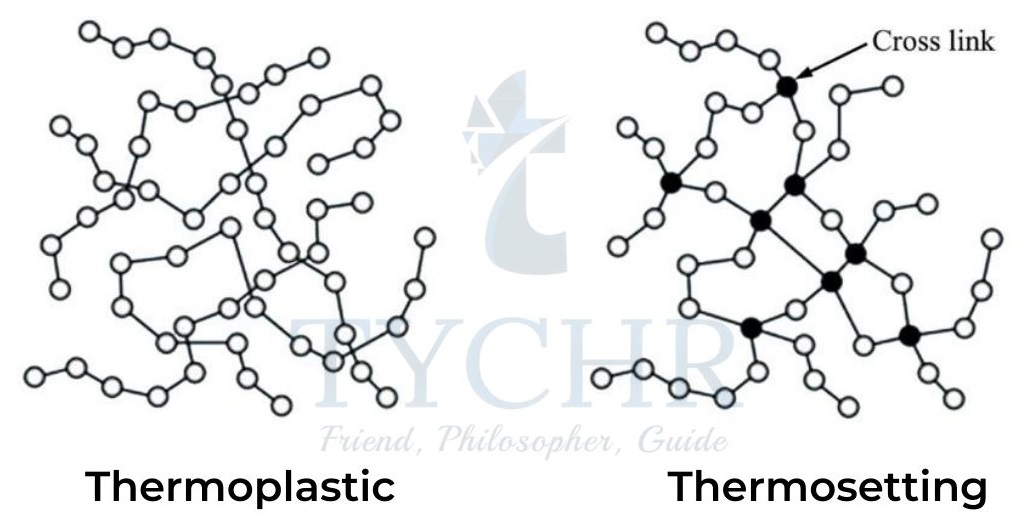
Thermoplastics and thermosets
Polymers can be classified as thermoplastics and thermosets based on their behaviour when heated.
Thermoplastics | Thermosets |
The molecules are in line or long chain with shorter entanglements. | The molecules are heavily cross- linked. |
Molecules are bound together by intermolecular (van der Waals’) forces. | Molecules are bound together by covalent bonds. |
When heated, the molecules move apart causing them to become untangled and soft and can be bent to any sorts of shapes. | When heated for the first time, they become permanently stiff and solid (on cooling). |
Can be repeatedly melted and reshaped through reheating. Heated softens cooled hardens and it’s a continuous process. | Cannot be reshaped and melted through reheating. Heated softens cooled permanently hard. |
Examples: Polythene, polystyrene, polyvinyl chloride(PVC) and polypropene etc. | Examples: Polysters, resins, epoxies, polyurethanes, and Bakelite etc. |
Table 3: Comparison between Thermoplastics and thermosets
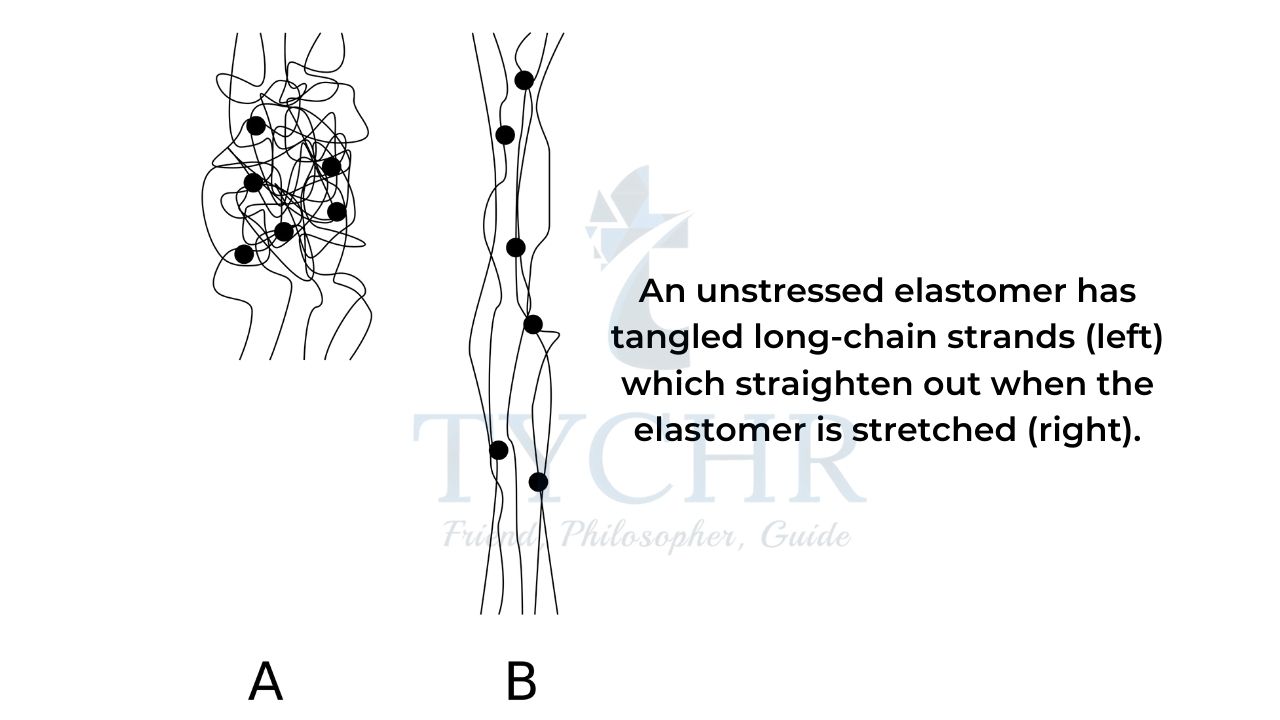
Elastomers
Elastomers are flexible polymers that return to their original shape after being deformed.
- Thermosets are used for manufacturing of elastomers because of their strength.
When the material is not under stress the polymer chain is tangled, loose, and flexible.
- Under stress the molecules assume a more linear form but retain their shape afterwards due to the covalently bonded cross-links.
Basically, elastomers are polymers having elastic properties, e.g. rubber.
PVC and the use of plasticizers
- Polyvinyl chloride (PVC), a synthetic resin made from the polymerization of vinyl chloride (chloroethene).
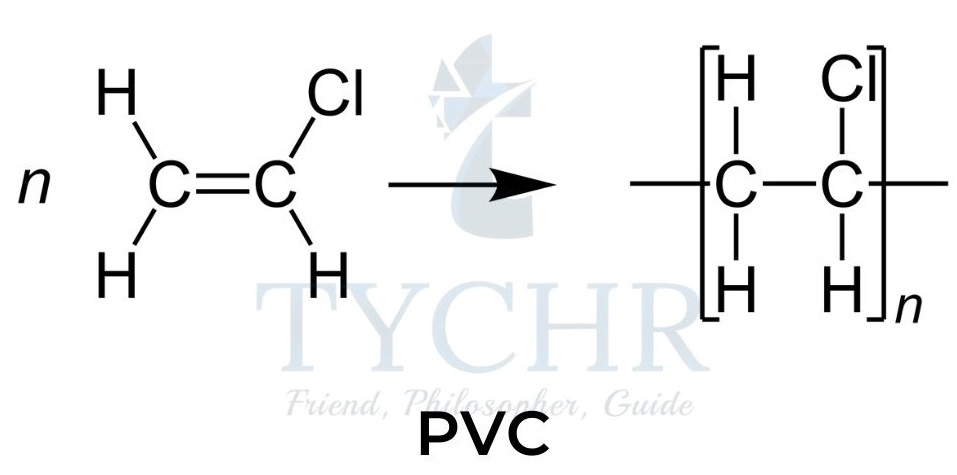
- This addition polymer was hard and brittle until Waldo Semon developed the technique of adding a plasticizer to the polymer.
- Plasticizers soften the PVC to make it flexible and bendable.
- PVC can only be rigid without plasticizers, like the PVC that is used in water pipes.
- Plasticizers work by embedding themselves between polymer chains, thus reducing the intermolecular forces between these chains.
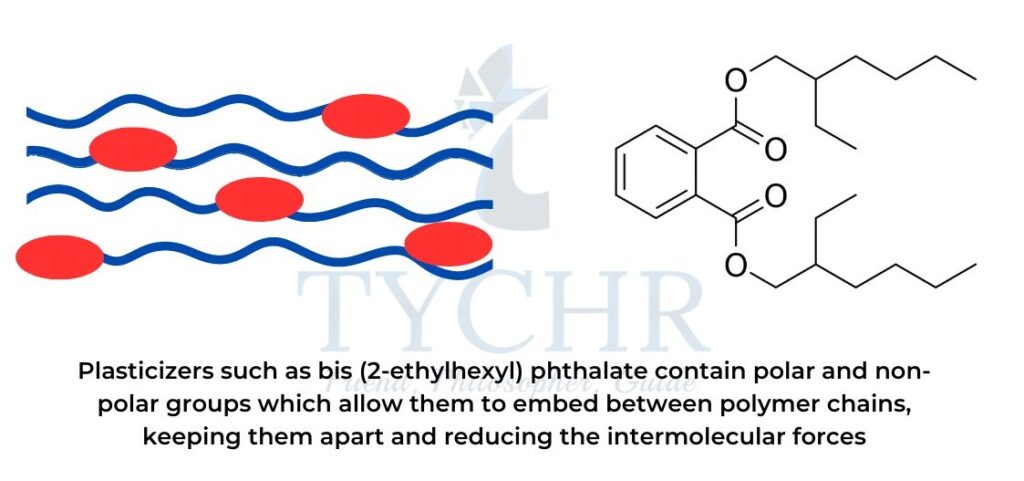
- The polar group locks the plasticizer in the polymer and the non-polar group weakens some of the attractive forces in the polymer chain, thus enhancing flexibility (refer to figure).
- Higher concentrations of plasticizer produce softer and more flexible polymers.
- The production of flexible PVC makes use of approximately 90% of all plasticizers.
- One of the first uses of plasticizers was to make PVC shower curtains. The majority is used in films and cables.
Polystyrene
- Polystyrene (polyphenylethene, (C8H8)n)is a thermoplastic polymer made from the monomer styrene.
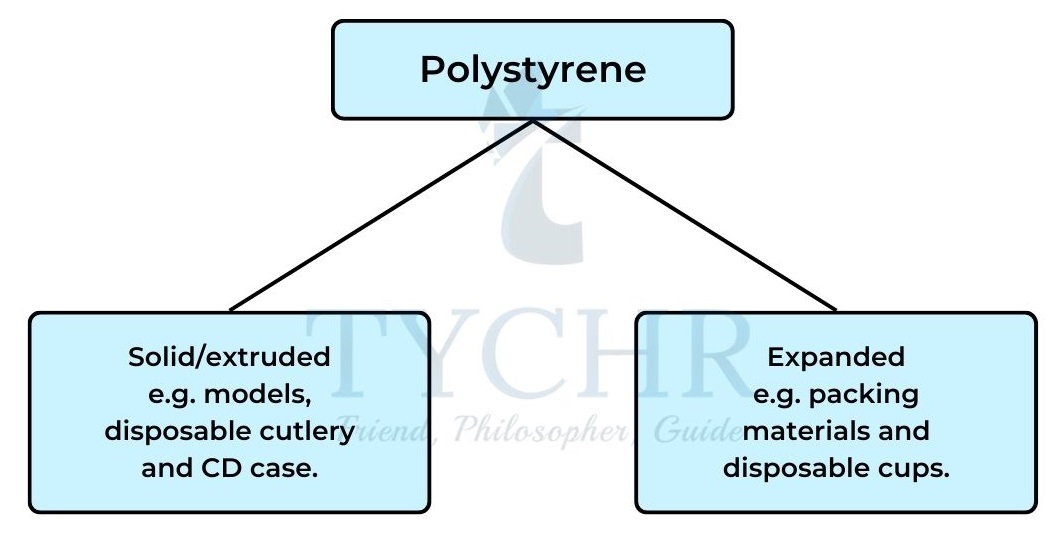
- Polystyrene can be solid or foamed. The solid plastic expands into a foam when heated by steam.
- Polystyrene is widely used in producing food containers and packaging.
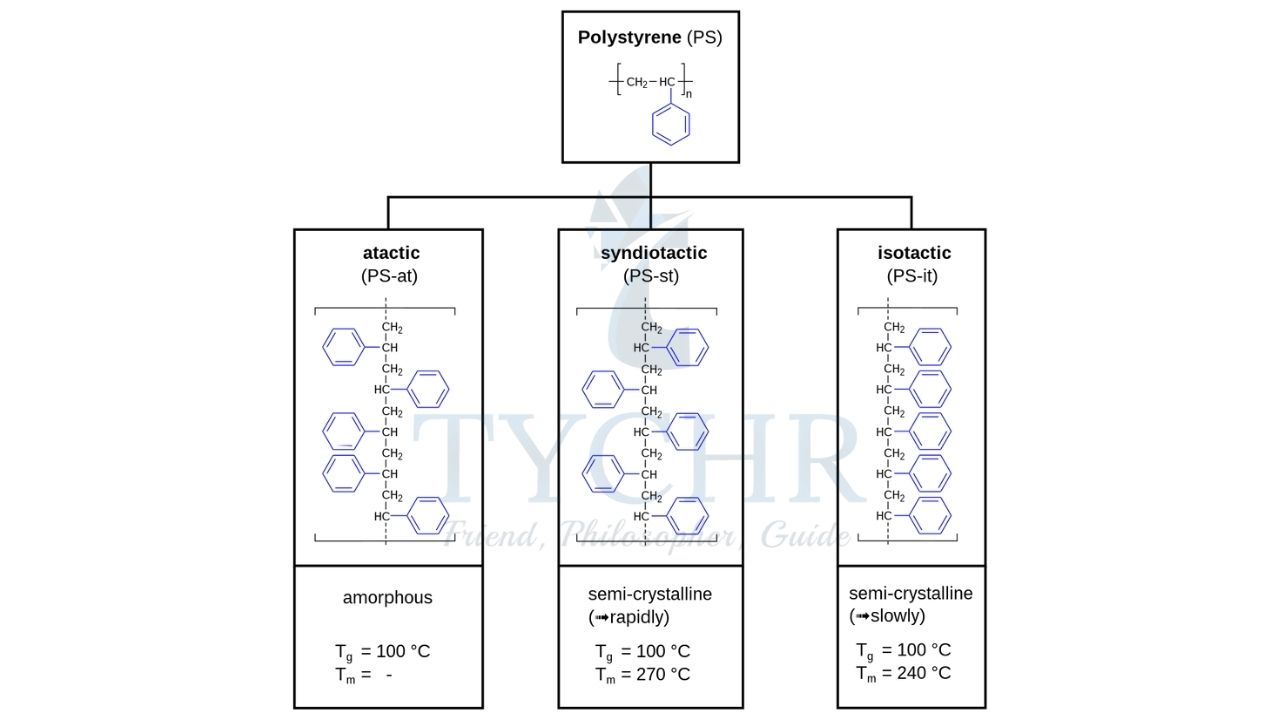
Isotactic, Atactic, and Syndiotactic addition polymers
Atactic Polymer | Isotactic Polymer | Syndiotactic Polymer |
An atactic polymer is a polymer material in which the substitutes in a carbon chain are arranged in a random manner. | An isotactic polymer is a polymer material which has the substitutes on the same side of the carbon chain. | Syndiotactic polymer is a polymer material which has the substitutes in an alternating pattern. |
Structure is mostly amorphous, softer than isotactic and syndiotactic polymers. | Structure is semi-crystalline, harder and rigid than atactic polymers. | Structure is crystalline i.e. harder and rigid than isotactic polymers. |
Identifying monomers
- You need to be able to identify up to three repeating units in a polymer.
- For example, a monomer of 2-methylpropene can undergo addition polymerization (Figure (h)) by cleavage of the double bond.
- The bond breaking is initiated by an acid (H+) catalyst.
- The repeating unit with alternating methyl and hydrogen substituents is favoured over having two methyl substituents next to each other.
Figure 18
Atom economy of polymerization reactions
- The amount of starting materials that result in useful or desired goods is measured by the atom economy.
% Atom economy = (molar mass of desired product/molar mass of all reactants )× 100% - Atom economy is a measure used in green chemistry, which takes into account not only the efficiency but also the degree of waste produced
- Efficient processes with a high atom economy are important in sustainable development as they create less waste and use fewer resources.
- The production of addition polymers represents 100% atom economy as all of the reactant monomer molecules end up in the product.
Nanotechnology
What is nanotechnology?
- Nanotechnology deals with the manipulation and control of atoms, molecules, and objects with dimensions of less than 100 nm (about 1000 atoms or less across).
- Chemical techniques place atoms in molecules using chemical reactions, whilst physical techniques allow atoms and molecules to be manipulated and positioned to specific requirements.
- There are two approaches to nano manufacturing: top-down or bottom up.
- The top-down approach reduces large pieces of material down to the nanoscale. Optical lithography, for example, uses short wavelengths of light (under 100 nm) in etching, such as in the design of integrated circuits. There is always some waste with the top-down approach as not all the material is used.
- The bottom-up approach uses molecular self-assembly of nanoparticles, in which molecules are selectively attached to specific surfaces.
- The principles of bimolecular recognition and self ordering are used to build up particles in perfect order without any external driving forces.
Constructing nanotubes
- Methods of producing nanotubes include arc discharge, chemical vapour deposition (CVD), and high pressure carbon monoxide disproportionation (HiPCO).
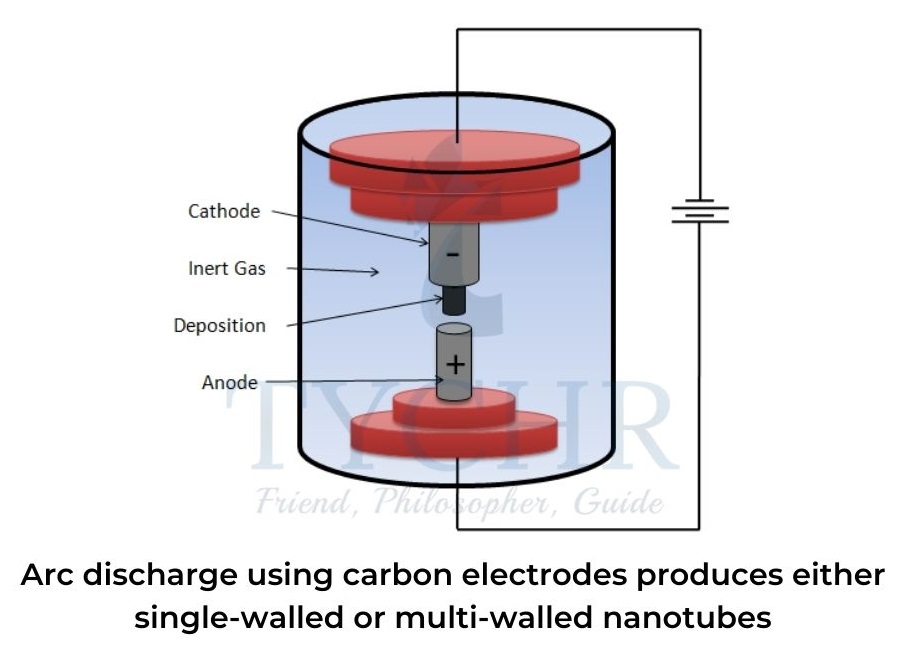
- Arc discharge was initially used to produce fullerenes, C60, and involves either vaporizing the surface of a carbon electrode or discharging an arc through metal electrodes submersed in a hydrocarbon solvent, forming a small rod-shaped deposit on the anode.
Arc discharge using carbon electrodes
- Two carbon rods are placed about 1 mm apart in a container of inert gas (helium or argon) at low pressure.Between the two electrodes, a direct current causes a high-temperature discharge that vaporizes a portion of one carbon anode and forms a small rod-shaped deposit on the other.
- If pure graphite is used, multi-walled nanotubes tend to be formed. Single-walled nanotubes have a diameter of 0.5–7 nm whereas multi-walled nanotubes have concentric tubes with an inner wall diameter of 1.5–15 nm and outer wall diameter of up to 30 nm.
Arc discharge using metal electrodes
- Electrodes of a metal such as nickel can be used for discharge in a hydrocarbon solvent, for example toluene (methylbenzene, C7H8) or cyclohexane (C6H12).
- The solvent is the source of carbon atoms for the nanotubes as the hydrocarbon is decomposed by the arc and soot is produced either at the anode (as occurs with toluene) or dispersed throughout the solvent.
Figure 20: Experimental apparatus for arc discharge using metal electrodes and a hydrocarbon solvent
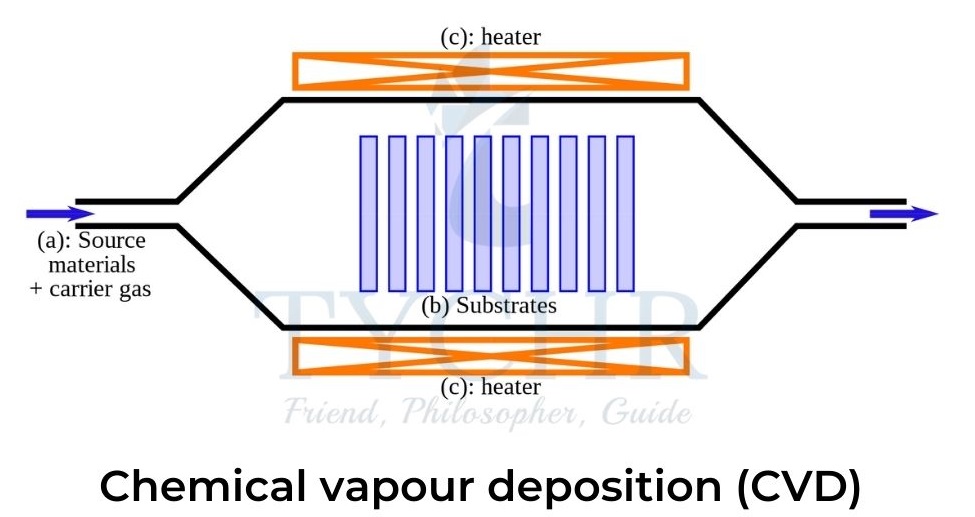
Chemical vapour deposition
- In CVD gaseous carbon atoms are deposited onto a substrate. This is achieved by the decomposition of a hydrocarbon gas such as methane or ethyne, or carbon monoxide over a transition metal catalyst.
- The covalent bonds in the gas are broken by either plasma discharge or heat, cracking the molecule, and the carbon atoms diffuse towards a substrate which is coated with a catalyst.
- The catalyst is usually iron, nickel, or cobalt and is attached to the substrate by heating or etching.
- One method of CVD is high pressure carbon monoxide disproportionation (HiPCO). In a disproportionation reaction the same substance is both oxidized and reduced.
- In HiPCO hot carbon monoxide is continuously supplied at high pressure into the reaction mixture.
- High temperature plasma rather than heating can be used to bring about CVD. A technique known as laser ablation uses a laser instead of an arc discharge to vaporize graphite.
- Either a continuous laser or pulses can be used and again single-, double-, or multi-walled nanotubes can be generated depending on conditions and the catalysts used.
Environmental impact: plastics
Challenges of materials science
- The design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances is referred to as “green chemistry,” and it is also referred to as “sustainable chemistry.”
- The design, production, and use of a chemical product are all affected by green chemistry.
- Plastics are polymers composed mainly of carbon and hydrogen. These have strong covalent bonds which are not easily broken so plastics do not decompose readily.
- Some polymers such as polyvinylchloride (PVC, polychloroethene) also contain chlorine and can release hydrogen chloride, HCl, or dioxins upon combustion.
- Other environmental concerns associated with plastics include the presence of volatile plasticizers.
Dioxins and PCBs
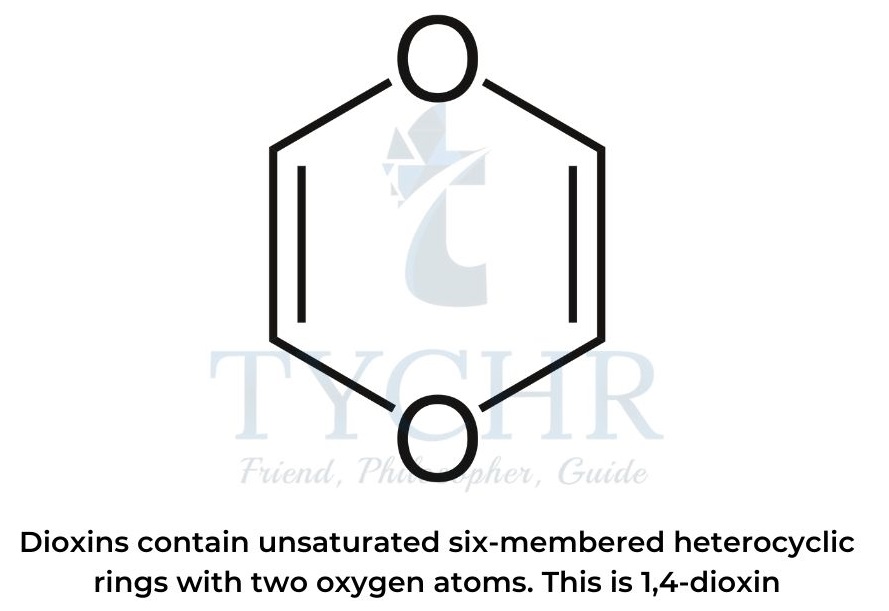
- The name “dioxins” refers to a class of environmental pollutants that are POPs.
- Dioxin molecules contain unsaturated six-membered heterocyclic rings with two oxygen atoms, usually in positions 1 and 4.
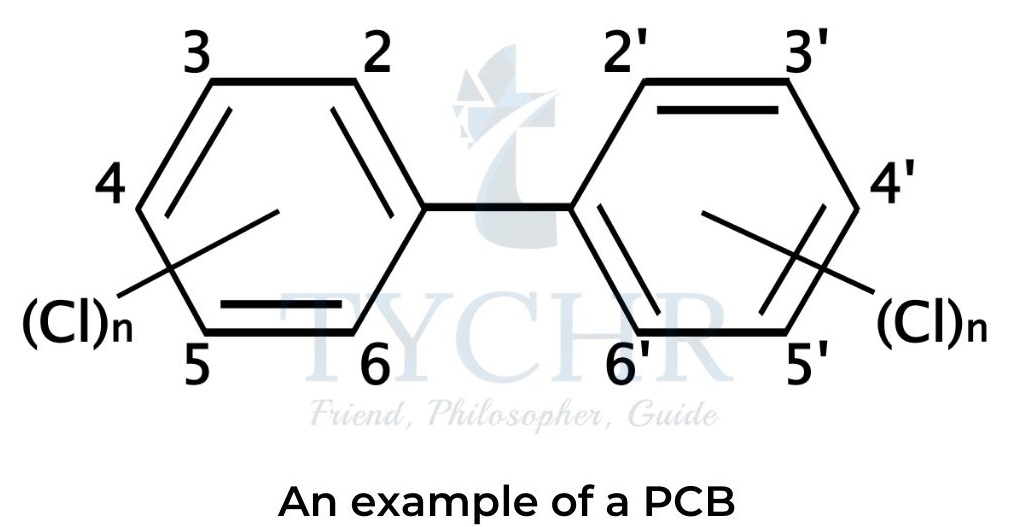
- Certain dioxin-like polychlorinated biphenyls (PCBs) with similar toxic properties are sometimes included in the term “dioxins”.
- TCDD is the most toxic of the more than 400 dioxin-related compounds that have been identified, but only about 30 of these are considered to have significant toxicity.
- Polychlorinated biphenyls (PCBs) are synthetic organic molecules containing two benzene rings with some or all hydrogen atoms replaced by chlorine.
- Dioxins are extremely carcinogenic—they cause cancer—and their concentration rises higher in the food chain as they accumulate in fat tissue.
- Dioxin-like substances act on a receptor present in all cells and can cause reproductive and developmental problems. They damage the immune system and interfere with hormone action.
Recycling of plastics
- Recycling rather than disposal is one of the most obvious ways of reducing environmental damage from any material. The atom economy increases while the need for the manufacture of new materials is reduced. Recycling of plastics, however, offers significant challenges. Thermosets cannot be melted down and recycled. Heating chlorine-containing polymers carries the risk of releasing dioxins so the method of remoulding needs to take account of this.
- Plastics are recycled based on their polymer type, identified by a resin identification code (RIC).
- Its primary purpose was the efficient identification of plastic polymer types, but it was soon applied to the classification of plastics for recycling.
- The number on the code gives information about the polymer type rather than its hardness, how frequently it can be recycled, difficulty in recycling, or colour.
- Recycling is an energy-intensive process. Plastic bottles for recycling need to be collected and separated from other material. The labels and any other debris are removed and the plastic is washed. It is automatically sorted using near-infrared scanning techniques and then manually checked again as incomplete sorting can lead to difficulties with the process.
- Some plastics cannot be recycled into new products. For example, the plastic cases of some cell phones contain bromine which is a fire retardant and these plastics cannot be put through a recycling process.

Condensation polymers
Condensation polymerisation
- Condensation polymers are polymers formed through a condensation reaction where molecules join together, losing small molecules as byproducts such as water or methanol.
- In condensation polymerization, many monomers are joined by condensation reactions to form the polymer.
- For two monomers to be joined by condensation polymerization they must each contain two functional groups, for example, a dicarboxylic acid and a diol:
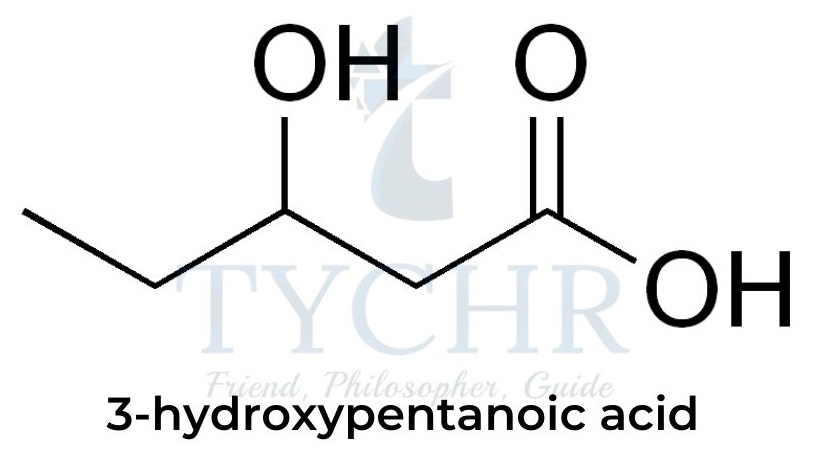
- The reaction can also proceed with only one monomer that contains two functional groups: for example, 3-hydroxypentanoic acid contains both an OH group and a COOH group so can polymerize with itself:
The Esterification reaction
- Acyl chlorides are a class of organic compounds in which the OH group of a carboxylic acid is replaced by a chlorine atom: R(C=O) Cl rather than R(C=O) OH.
- Acyl chlorides react with amines rather than alcohols in a condensation reaction that forms an amide.
- For example, ethanoyl chloride, CH3COOH and methylamine, CH3NH2 react to form N- methylethanamide, CH3NHCOCH3.
- A hydrogen from the amine and the OH group from the acid condense to form hydrogen chloride.
- The mechanism is the same as for the esterification reaction except that there is an N rather than an O next to the carbonyl group forming an amide linkage.
Phenol- methanol plastics
- Another example of condensation polymer is Phenol–methanal plastics.
- Step 1: electrophilic substitution (see topic 20) of a hydrogen atom at the benzene ring with methanal:
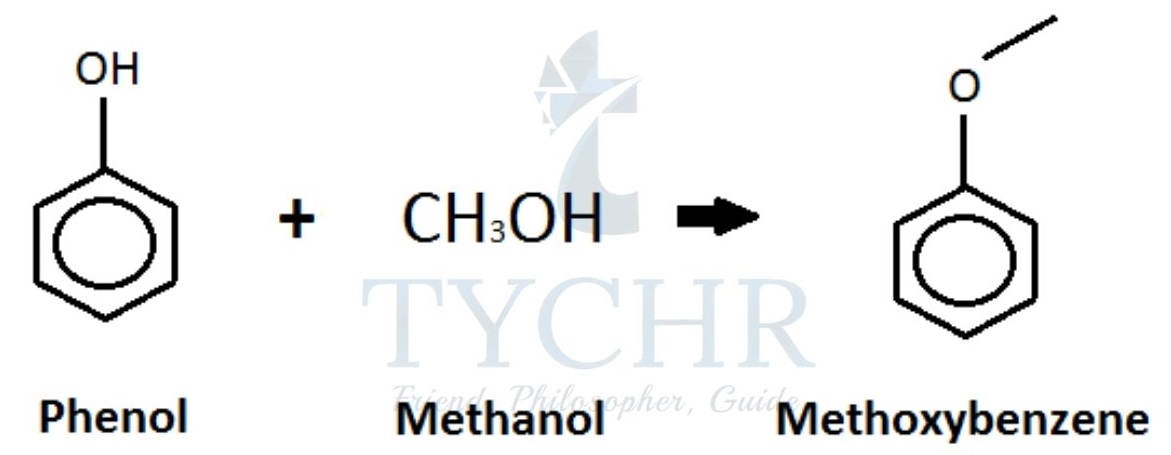
- The OH group in phenol is an ortho–para director, meaning that substitution of the hydrogen will occur on the number 2 (ortho) or number 4 (para) carbon atom in the benzene ring.
- Step 2: condensation step
Figure 29
- And this is a continuous process with substitution occurring in either the 2- and/or the 4- position depending on the ratio of methanal to phenol.
- Phenol–methanal polymers are thermoset plastics that have the ability to withstand high temperatures and electric fields, they are used as electrical insulators in construction and brake linings in vehicles.
Polyurethanes
- Polyurethanes are another type of condensation polymer, a polyamide:
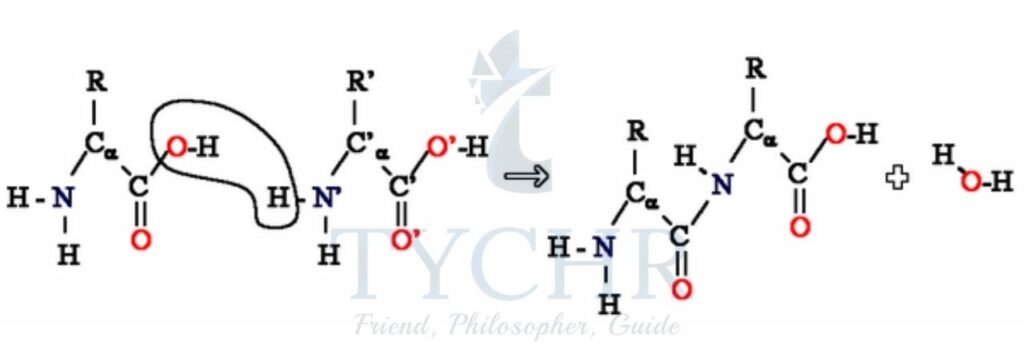
- They form foams such as those used in padded chairs, elastomers used in paint, and fibres to produce spandex (elastane), a synthetic fabric with elastic properties.
- Monomers used to form polyurethanes are often a diol or diamine and a dicyanate (cyanates have the N=C=O functional group).
Breaking down condensation polymers
- Condensation polymers are formed from two monomers, releasing a small molecule in the process.
- These polymers can be broken down by the reverse reaction.
- Polyamides with strong hydrogen bonding such as Kevlar can dissolve in sulfuric acid: the acid donates a proton to the oxygen and nitrogen atoms involved in hydrogen bonding.
- This breaks hydrogen bonds between chains of Kevlar fibres and the substance dissolves.
Figure 31: Strong hydrogen bonds between polymer chains in Kevlar. Care must be taken to avoid interfering with hydrogen bond formation during production; for example the solvents must be free of ion impurities
- Nylon, another polyamide, reacts readily with dilute acids in a hydrolysis reaction.
- In breaking down amides to amines and carboxylic acids the condensation product, water, must be added and the reaction proceeds faster at high temperatures.
Summary of polymer properties
Structural property | Physical property | Examples |
Chain length | The longer the chain, the stronger the polymer. | Longer polymer chains have higher melting point, increased strength, and increased impact resistance due to increased van der Waals’ forces. |
Branching and packing structures | Straight unbranched chains can pack more closely. A higher degree of branching keeps strands apart and weakens intermolecular forces | HDPE with no branching is more rigid than the more branched LDPE. Use of plasticizers in PVC to soften the polymer. |
Side groups on monomers | Hydrogen bonding can increase strength, e.g. Kevlar. Atactic and isotactic placement can influence strength, e.g. polystyrene | Polystyrene |
Cross-linking | Extensive covalently bonded cross-linkage increases polymer strength | Vulcanized rubber, Bakelite |
Table 4: Summary of polymer properties
Environmental impact: heavy metals
Applications of heavy metals
- “Heavy metals” is a term that refers to toxic metals such as lead, mercury, and cadmium which have cumulative effects on health.
- Such metals have many uses: lead, nickel, and cadmium are used in batteries; arsenic, bismuth, and antimony are often found in semiconductors; and mercury has many uses including in instruments such as thermometers, barometers, and diffusion pumps and has been used in mining, amalgams, and manufacturing.
- Heavy metals accumulate in biological systems over time. They are stored in living organisms and passed on in the food chain.
- Toxic metals can react with enzyme binding sites and inhibit or over-stimulate these enzymes. For example, cadmium belongs to the same group as zinc, and competes with zinc during absorption into the body.
- Through a variety of mechanisms, toxic doses of transition metals can disrupt the normal oxidation–reduction balance in cells.
- They can disrupt the endocrine system because they compete for active sites of enzymes and cellular receptors.
- Their ability to form complex ions enables them to bind with enzymes: iron, for example, forms a complex with haemoglobin which is essential for oxygen transport.
- Finally, transition metals are very good catalysts.
Chelating effects
- Apart from the Fenton reaction, other methods of removing heavy metals include precipitation, adsorption, and chelation. Chelation takes advantage of a metal’s ability to form complex ions.
- Chelating agents are used to remove heavy metals such as lead, arsenic, and mercury from the body. Once chelated the complex ion is too large to enter cells but being an ion is water soluble so can be excreted from the body.
- Polydentate ligands such as EDTA are usually more effective than monodentate ones and will replace them in reactions.
Adsorption of heavy metals
- Another method of removing heavy metals is by adsorption onto a solid surface.
- There are many methods including activated carbon, charcoal filters and clays.
- Biomass such as brewer’s yeast has also been found to be effective.
- Ion-exchange mechanisms which exchange heavy metal ions for calcium or sodium ions can also remove heavy metal contaminants.
- The treated water then undergoes further purification processes such as ultraviolet treatment to kill bacteria.

