option b biochemistry Notes
Introduction to biochemistry
What is biochemistry?
- Biochemistry studies chemical processes in living cells at the molecular level.
- Biochemical processes, collectively known as metabolism, are very complex and involve many chemical reactions occurring in the same place and at the same time.
- Some of these reactions (anabolic reactions) produce large organic molecules from simpler organic or inorganic substances while in other reactions (catabolic reactions), complex molecules are broken down into smaller fragments.
What drives metabolism?
- Anabolic reactions increase the complexity and order of biochemical systems and thus reduce their entropy.
- Such processes cannot be spontaneous; they require energy, which is supplied by catabolic reactions or in photosynthesis is received in the form of light from the sun.
- Photosynthesis is the major source of energy for green plants and some bacteria.
- Other organisms, including humans, rely entirely on the chemical energy obtained from food by a complex set of metabolic processes known as respiration.
Molecules of life
- The primary chemical element in all biologically important molecules is carbon. Its relatively small size, moderate electronegativity, and the electronic configuration of the outer shell (2s22p2 in the ground state and 2s12p3 in the excited state) allow carbon to form up to four single or multiple covalent bonds with many elements, including itself.
- The energies of these bonds are high enough to produce stable molecules and at the same time low enough to allow such molecules to undergo various transformations.
- This combination of stability and reactivity makes organic molecules the chemical basis of life.
Photosynthesis, respiration, and the atmosphere
- Photosynthesis and respiration are responsible for the global balance of oxygen and carbon dioxide.
- Nearly all the oxygen in the Earth’s atmosphere and oceans is a by-product of photosynthesis, the process that started in blue-green algae (cyanobacteria) over two billion years ago and dramatically changed our planet.
- Along with the production of oxygen, photosynthesizing bacteria consumed most of the atmospheric carbon dioxide and made the Earth habitable for higher life forms, such as plants, humans, and other animals.
- Biochemical studies allow us to predict the impact of these changes on metabolism, life cycles, and ultimately on the survival of various organisms, including our own species.
Proteins and enzymes
The central role of proteins in biochemistry
- Proteins are the most diverse and abundant class of biopolymers, responsible for over 50% of the dry mass of cells.
- This fact reflects the central role of proteins in metabolic processes, transport and sensory functions, structural integrity, and virtually all other molecular aspects of life.
- Simple proteins are linear polymers of 2-amino acids.
- The structural units of proteins are joined together by amide linkages (also known as peptide bonds) in strict order and orientation. Most proteins contain several hundred to several thousand structural units.
- Shorter polymers composed of less than 20 residues of 2-amino acids are called peptides.
Naming peptides
- The order of amino acid residues in peptides is very important for example, the dipeptides Ala-Ser and Ser-Ala are two different compounds that might have very different physiological properties.
- The first amino acid in a peptide has a free NH2 group, described as N-terminal, while the last amino acid has an unreacted COOH group (C-terminal).
- Both N- and C-terminals can participate in further condensation reactions that produce larger peptides and proteins.
- In living organisms the synthesis of peptides usually begins from their N-terminals, so the sequence of amino acids is traditionally recorded in the same way.
Properties of peptides
- The acid–base properties of peptides are similar to those of 2-amino acids.
- Terminal NH2 and COOH groups, together with the functional groups of the peptide side-chains, can be ionized to various extents and, depending on the pH of the solution, produce polyions with multiple positive and negative charges.
- Each peptide has a characteristic isoelectric point (pI), which can be used to separate and analyse peptide mixtures by gel electrophoresis. Together with proteins and individual amino acids, peptides act as acid–base buffers and maintain a constant pH of biological fluids.
Proteins: Primary structure
- Proteins are the most diverse biopolymers that vary greatly in size, shape, and composition. Simple proteins consist of a single chain of 2-amino acid residues connected to one another in strict order and orientation.
- The exact sequence of amino acid residues joined together by peptide linkages is known as the primary structure of a protein. Similar to peptides, proteins have N- and C-terminals, and the primary structure is traditionally written from left to right starting from the N-amino acid.
Proteins: Secondary structure
- Long chains of amino acid residues in proteins tend to adopt certain highly ordered conformations, such as α-helix and β-pleated sheet.
- These local and regularly repeating conformations are stabilized by intramolecular hydrogen bonds between carbonyl and amino fragments of peptide linkages and are collectively known as the secondary structure of a protein.
Enzymes
- Most proteins in the human body act as enzymes – highly specific and efficient biological catalysts that control virtually all biochemical processes, from the digestion of food to the interpretation of genetic information.
- Molecules that are modified by enzymes are called substrates.
- Enzymes are large molecules, and the substrate interacts with a relatively small region of the enzyme known as the active site.
- The catalytic process begins when the substrate comes into close proximity with the active site. If the substrate and the active site have complementary structures and correct orientations, a chemical “recognition” occurs and an enzyme– substrate complex is formed.
- Multiple intermolecular interactions in this complex distort and weaken existing chemical bonds in the substrate, making it more susceptible to certain chemical transformations within the active site.
- The catalytic cycle completes when the reaction product detaches from the enzyme, leaving the active site available for the next substrate molecule.
Lipids
Lipids in living organisms
- Lipids are a broad group of naturally occurring substances that are largely non-polar and therefore insoluble in water.
- Unlike other classes of biomolecules, lipids are defined in terms of their properties rather than structure or chemical behaviour.
- In living organism’s lipids perform various functions, including energy storage, chemical messaging and transport, thermal insulation of the body, and physical separation of the cell content from biological fluids.
- Most lipids are relatively small and predominantly hydrophobic molecules that tend to form large assemblies with regular structures.
- However, in contrast to covalently bonded subunits of biopolymers, individual molecules of
lipids in such assemblies are held together by weak van der Waals’ forces.
Fatty acids and triglycerides
- Fatty acids is a common name for long-chain unbranched carboxylic acids.
- Saturated fatty acids contain only single carbon–carbon bonds and have the general formula CnH2n+1
- Unsaturated fatty acids with one or more CH=CH groups in their molecules are described as monounsaturated and polyunsaturated, respectively.
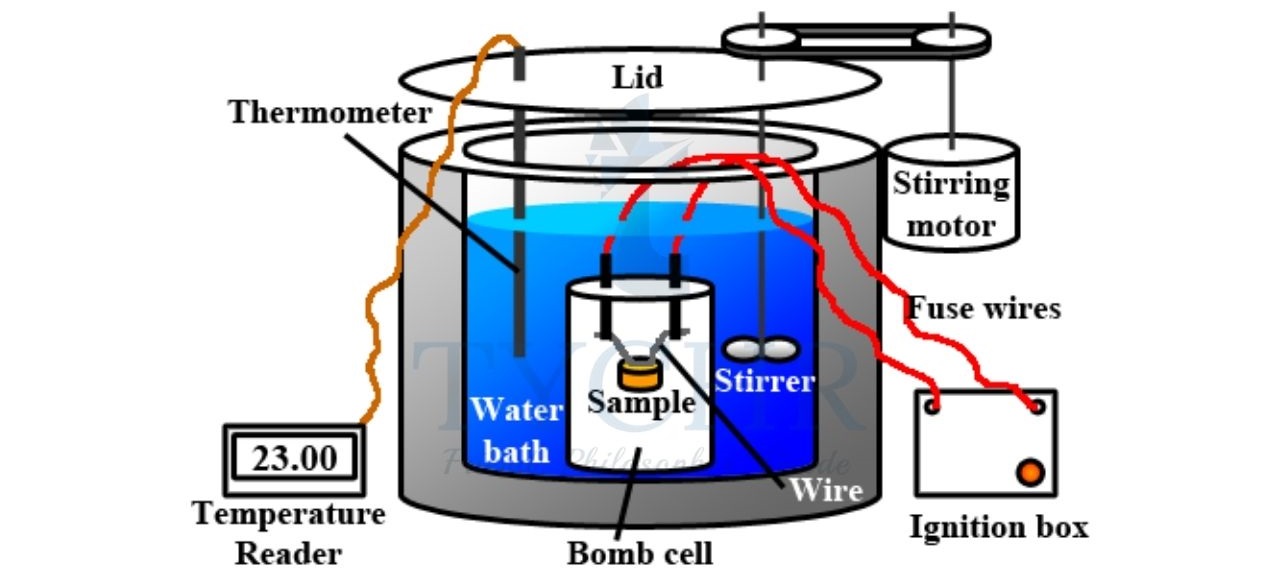
Calculating energy content
- The energy value of a food can be either calculated from the percentages of its main ingredients or determined experimentally using a calorimeter.
- An outline of a simple bomb calorimeter is shown in figure.
- If a food sample of a known mass is mixed with oxygen and combusted in the reaction chamber of the bomb calorimeter, the released energy is absorbed by the water, and can be calculated from the temperature change, the mass of water and the heat capacity of the calorimeter itself.
- Alternatively, a series of samples with known combustion enthalpies can be used for plotting a calibration curve, from which the combustion enthalpies of food samples can be determined.
Steroids
- Steroids are a class of lipids with a characteristic arrangement of three six-membered and one five-membered hydrocarbon rings fused together in a specific order.
- The carbon atoms in this four-ring structure, known as the steroidal backbone, are traditionally numbered as shown in figure.
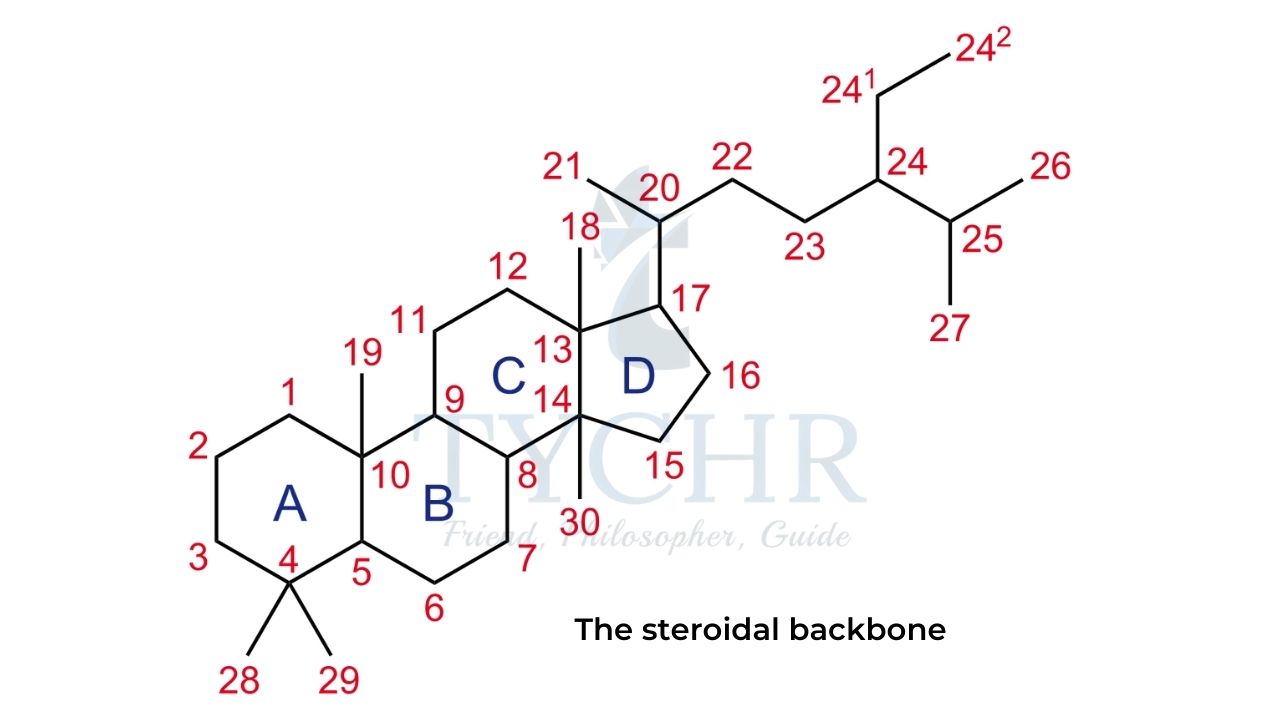
- Cholesterol is an essential component of cell membranes and the main precursor of all steroidal hormones produced in the human body.
- In cell membranes, the hydroxyl groups of cholesterol molecules hydrogen bond to phosphate groups of phospholipids while the non-polar hydrocarbon backbone and the substituent at the five-membered ring form van der Waals’ interactions with the fatty acid residues.
- As a result, embedded cholesterol molecules increase the rigidity of cell membranes and regulate their permeability to metabolites.
- Since cholesterol is largely hydrophobic, its solubility in blood plasma is extremely low. In the human body cholesterol is transported as a component of lipid–protein complexes known as lipoproteins.
- Depnding on their composition and density these complexes are classified as low-density lipoproteins (LDL) or high-density lipoproteins (HDL).
- Generally, the density and solubility of lipoproteins in water decrease with increasing lipid content, so the amount of cholesterol carried by LDLs is significantly higher than that by HDLs.
Steroid hormones
- Besides cholesterol, several hundred other steroids with various biological functions are known. In the human body all steroids are synthesized from cholesterol, which loses its side- chain at carbon 17 and undergoes a series of enzymatic transformations.
- Most steroids are hormones – the chemical messengers that regulate metabolism and immune functions (corticosteroids), sexual characteristics and reproductive functions (sex hormones), or the synthesis of muscle and bone tissues (anabolic steroids).
- The male sex hormones are produced in the testes and include testosterone and androsterone:
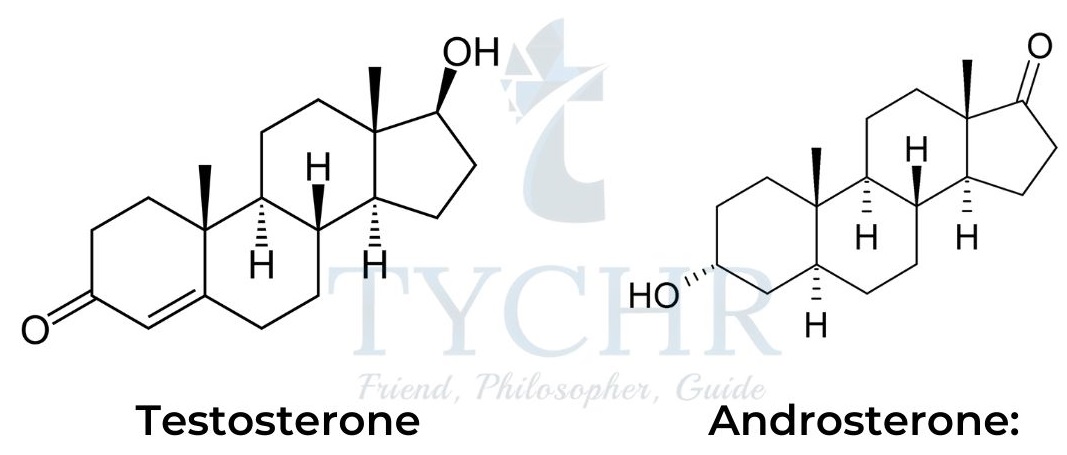
- In addition to androgenic functions (the development of male sex characteristics), male sex hormones act as natural anabolic steroids.
- The female sex hormones are produced in the ovaries and include progesterone and estradiol:
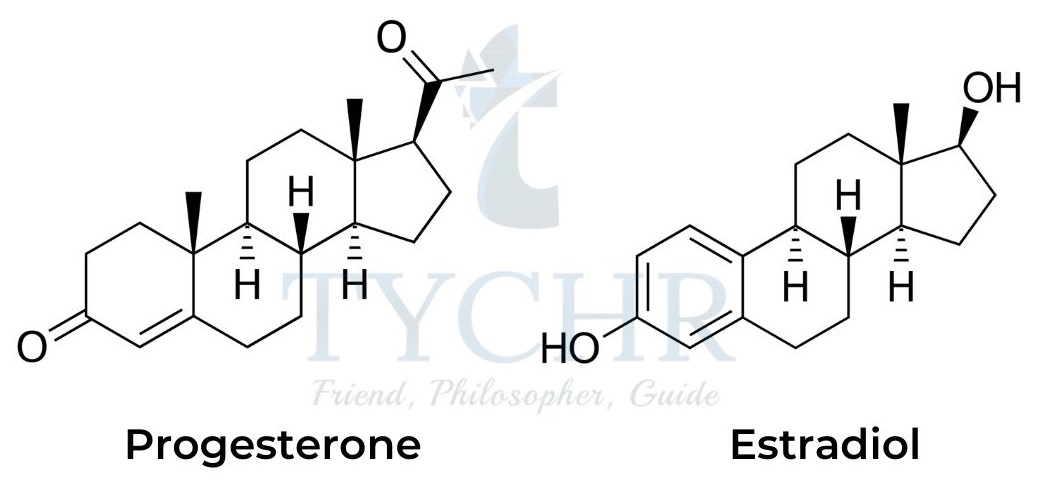
- Estradiol and progesterone are responsible for controlling sexual development and menstrual and reproductive cycles in women. Estradiol is one of the few steroids that contains an aromatic ring in the steroidal backbone.
Carbohydrates
Introduction to carbohydrates
- Carbohydrates are a family of oxygen-rich biomolecules that play a central role in the metabolic reactions of energy transfer.
- Most carbohydrates have the general formula Cn(H2O)m (“hydrates of carbon”) although this term is also used for deoxyribose (C5H10O4) and other structurally similar compounds.
- Monosaccharides with five and six carbon atoms in their molecules are known as pentoses and hexoses, respectively.
- For example, glucose and fructose are hexoses while ribose and deoxyribose are pentoses.
- If the carbonyl group is connected to the terminal carbon atom the monosaccharide belongs to the class of aldehydes and is called an aldose (“aldehyde sugar”).
- Similarly, monosaccharides with a carbonyl group at the second carbon atom are known as ketoses (“ketone sugar”).
The importance of glucose
- Glucose is the most common monosaccharide that occurs in all living organisms.
- It is the main product of photosynthesis and the primary source of energy for cellular respiration.
- Glucose is an important intermediate in various metabolic processes including the synthesis of mono-, di-, and polysaccharides, amino acids, vitamins, and many simple biomolecules such as 2-hydroxypropanoic (lactic) acid or ethanol.
- The latter compound is produced from glucose in an enzymatic process known as alcoholic fermentation:
C6H12O6 → 2CH3CH2OH + 2CO2 - In addition to its use in alcoholic beverages, ethanol is increasingly used as a component of biofuels, reducing consumption of fossil fuels and the net emission of greenhouse gases.
Glycogen and cellulose
- In the human body the short-term energy store is in the form of glycogen, which is structurally similar to amylopectin but is more densely branched and contains up to a million glucose residues. Glycogen is concentrated in liver and muscle tissue where it is hydrolysed into glucose when the energy is needed.
- Another condensation polymer of glucose, cellulose, is the major structural polysaccharide in plants and an important component of a healthy diet (dietary fibre).
Vitamins
Introduction to vitamins
- Vitamins are organic micronutrients that cannot be synthesized by the organism in sufficient amounts and must either be obtained from suitable foods or taken as food supplements.
- A lack (deficiency) of vitamins leads to various health conditions and in some cases can be fatal, even if all other food constituents (proteins, fats, carbohydrates, minerals, and water) are present in the diet.
Preventing deficiencies
- To prevent the adverse health conditions associated with vitamin deficiencies, humans must receive vitamins on a regular basis.
- The optimal frequency of the intake of different vitamins depends on their chemical structures and the way they are distributed and stored in the body.
- Water-soluble vitamins such as vitamin C and some group B vitamins concentrate in blood plasma and intracellular fluids.
- These vitamins have relatively short half-elimination times, from 30 minutes to several weeks, so they should be supplied to the body on a daily basis.
- In contrast, fat-soluble vitamins such as vitamins A and D are accumulated in the liver and fat tissue, where they can be stored for prolonged periods of time (up to several months).
- These vitamins can be consumed less frequently without any detrimental health effects.
Three important vitamins
Vitamin A: Retinoids and carotenes
- The collective name “vitamin A” refers to several organic compounds, retinoids and carotenes, that perform similar functions in the human body. The structure of one of these compounds, retinol.
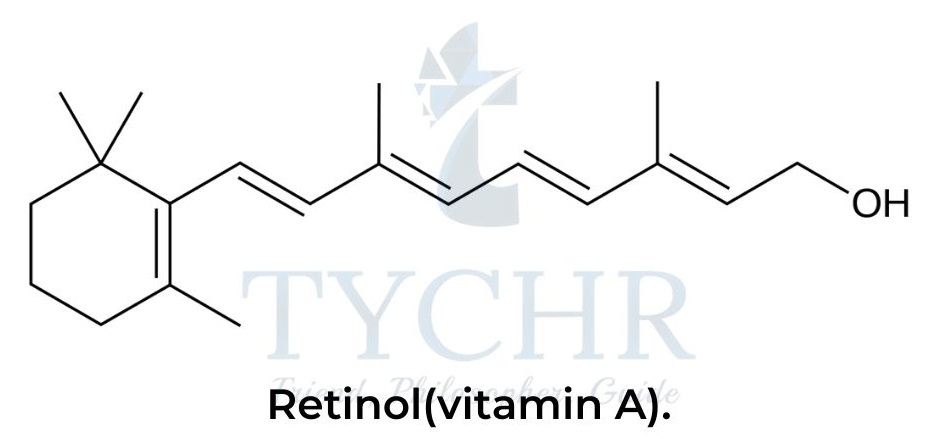
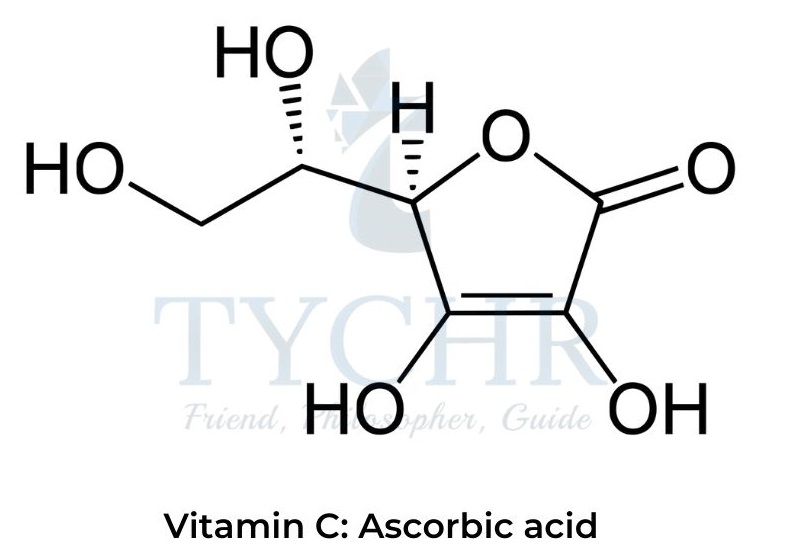
Vitamin C: Ascorbic acid
- Vitamin C or ascorbic acid is a relatively simple oxygen-rich organic molecule containing multiple polar functional groups.
- Several hydroxyl groups and an ester fragment in the molecule can form multiple hydrogen bonds with water, making it a water-soluble vitamin.
- The same polar functional groups make ascorbic acid insoluble in fats, so it cannot be stored in the body for a long time and requires regular intake.
- Ascorbic acid is a powerful antioxidant and reducing agent capable of donating one or two electrons in biochemical redox reactions, for example:
Vitamin D: Cholecalciferol
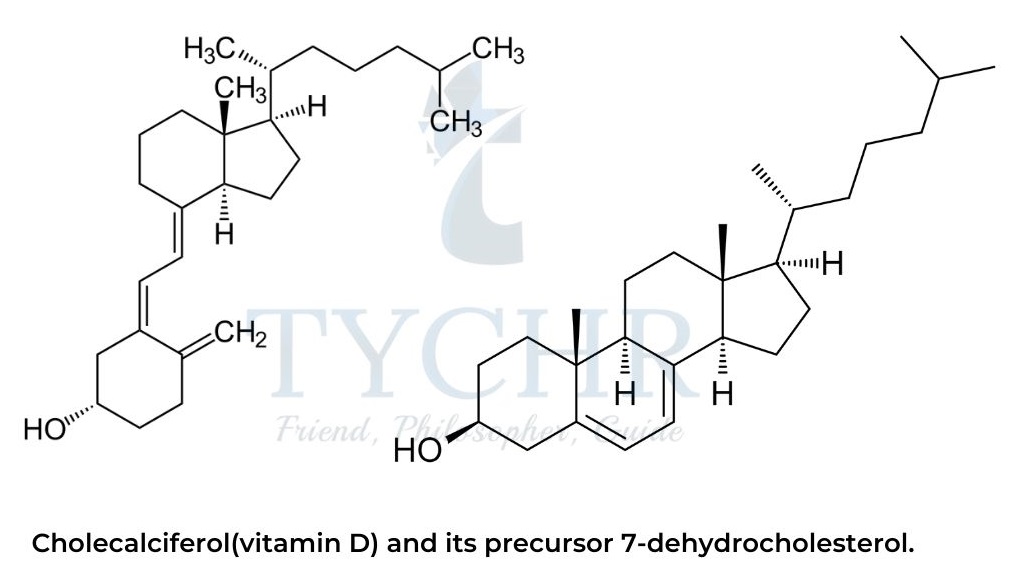
- The collective name “vitamin D” refers to cholecalciferol and three other structurally similar organic compounds with a partly broken steroidal backbone.
- The biosynthesis of cholecalciferol takes place in the skin and requires ultraviolet (UV) light (which is present in the sunlight spectrum) to open the second six-membered ring of 7-dehydrocholesterol.
- The human body is normally able to produce enough vitamin D to meet its own metabolic requirements; however when exposure to sunlight is limited (especially at high latitudes during the winter), vitamin D becomes an essential micronutrient that must be obtained from the diet.
Decomposition of vitamins
- Vitamins are complex organic compounds and therefore may undergo various chemical transformations when exposed to heat, light, and atmospheric oxygen.
- The hydrocarbon backbones of fat-soluble vitamins such as A and D are relatively stable to heat and do not decompose significantly when the food is boiled or steamed.
- In contrast, water-soluble vitamin C is unstable at high temperatures and can be lost by leaching from foods into cooking water.
- Overcooked or fried foods can lose more than 50% of their fat-soluble vitamins and nearly all their vitamin C.
Biochemistry and the environment
The nature of biochemistry
- Biochemistry is a multidisciplinary science that studies the chemical changes associated with living organisms and their interactions with the environment.
- Our increasing understanding of biochemical processes has greatly enhanced our ability to control biological systems but at the same time created serious ecological problems and raised our awareness of the environmental and ethical implications of science and technology.
Xenobiotics
- The rapid development of organic chemistry in the twentieth century led to the industrial production of pesticides, medicinal drugs, and other chemical compounds that had no natural sources and therefore were foreign to living organisms.
- These compounds, known as xenobiotics, are generally toxic to various life forms and are more resistant to biodegradation than naturally occurring organic molecules. Certain xenobiotics (persistent organic pollutants, POPs) can remain in the soil and in animal fatty tissues for many decades after their release into the environment.
Host–guest complexes
- Although enzymatic processes are highly selective and efficient, many enzymes are unstable in the environment and show their optimal activity in narrow ranges of pH and temperature.
- Certain synthetic molecules are free from these limitations and can selectively bind to environmental pollutants.
- The resulting supramolecules, or host–guest complexes, mimic the structures of enzyme– substrate complexes, where the synthetic analogue of the enzyme (host) and the environmental pollutant (guest) are held together by multiple non-covalent interactions including van der Waals’ forces, ionic bonds, and hydrogen bonds.
Plastics and polymers
- Non-biodegradable materials such as plastics and other synthetic polymers are the most abundant and persistent environmental pollutants produced by humans.
- The accumulation of plastic waste is not only unsightly but presents a serious danger to living organisms, especially birds and marine animals.
- Entanglement and ingestion of non-biodegradable materials reduce the mobility and interfere with the digestive functions of affected species, which often leads to starvation and death.
- In a recent study over 95% of sea birds were found to have plastic objects in their stomachs, which in some cases prevented the birds from flying due to additional weight and chronic malnutrition.
Proteins and enzymes
Acid–base properties of 2-amino acids
- Depending on the solution pH, the carboxyl and amino groups in these amphoteric compounds can be ionized to various extents producing ionic species with different charges.
- In strongly acidic solutions, amino acids and proteins are protonated and exist as cations while in strongly alkaline solutions, deprotonation occurs and anions are formed.
- At a certain pH known as the isoelectric point (pI) and specific to each amino acid or protein, the positive and negative charges of ionizable groups cancel one another, producing zwitterions with net zero charges.
Buffer pH range
- Amino acids can act as acid–base buffers only within certain pH ranges, where both components of a conjugate acid–base pair are present in the solution at sufficient concentrations.
- At pH = pKa1 and pH = pKa2, the amino acid reaches its maximum buffer capacity and can neutralize the greatest amount of strong acid or base before any significant pH change occurs.
Proteins as biological buffers
- Similar to amino acids, proteins can exist in cationic, zwitterionic, and anionic forms due to the presence of ionizable side-chains in their constituent amino acid residues.
- These side-chains form various polyions that act as biological acid–base buffers.
- The exact amino acid composition of a protein usually correlates with the pH of the biological fluid where this protein occurs.
- In each case, the protein buffers play an important role in maintaining a constant pH of biological fluids, which is essential for the integrity of body tissues and enzyme functions.
Protein assay
- The detection of proteins and the determination of their concentrations in solutions, known as protein assay, are the most common analytical procedures in biochemical experiments.
- In modern laboratories protein assays often involve absorption spectroscopy in the ultraviolet and visible regions of the electromagnetic spectrum.
- This technique, often referred to as UV-vis spectrometry, measures the absorption of UV and/or visible light by proteins or their complexes with organic dyes and transition metal ions.
- Certain organic dyes such as Coomassie® Brilliant Blue bind to arginine and aromatic residues and form highly conjugated systems of delocalized electrons with maximum absorption at 595 nm in the orange region of the visible spectrum.
- The complexes of proteins with transition metal ions also absorb visible light due to d-orbital electron transitions.
- A typical UV-vis spectrophotometer consists of a light source that produces UV and visible light, a mono chromator that allows only a narrow bandwidth of light to pass through, a cuvette that holds the studied sample, a detector and amplifier that convert the light into an electric current and a digital output device or computer that allows analysis of the experimental results.
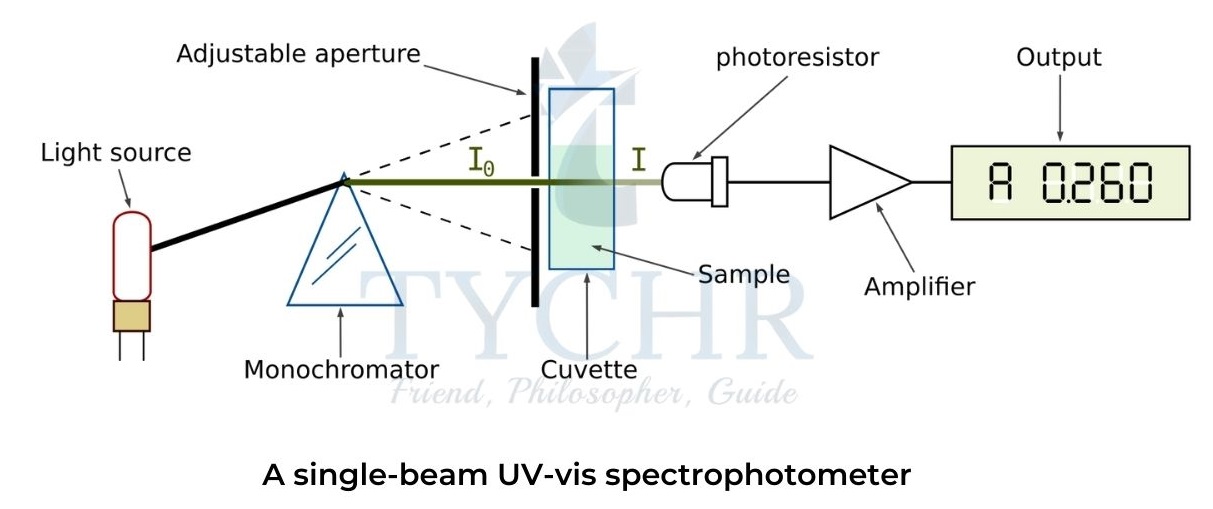
- The sample solution containing a protein is put into a transparent cuvette and placed inside the spectrophotometer.
- Depending on the protein concentration and experimental conditions, the intensity of UV or visible light passed through the sample will be reduced to some degree.
- The logarithmic ratio between the intensity of light emitted by the monochromator (I0 ) and the intensity of light passed through the sample (I) is known as the absorbance (A) of the sample:
A = log(𝐼0/𝐼)
Other analytical techniques
- Although UV-vis spectra provide some information about the structure of organic compounds, the identification of proteins on the basis of their UV-vis spectra alone is problematic because the spectra of different proteins are similar to one another and highly sensitive to experimental conditions.
- However, such identification becomes possible when a UV-vis spectrophotometer is used as a detector in high-performance liquid chromatography.
- In this case, the proteins in the sample are first separated chromatographically and then the UV-vis spectrum of each protein is matched to a large library of known compounds.
- Unidentified components of the mixture can be further analysed by various techniques including gel electrophoresis, high resolution NMR, and mass spectrometry.
Nucleic acids
Heredity and the storage of biological information
- Every living organism contains many thousands of proteins with strictly defined structures and functions.
- The amino acid sequences of specific proteins in all the cells of a particular organism are identical and differ only slightly between individuals of the same species.
- This fact suggests that there must be a certain mechanism that allows cells to store and interpret biological information, as well as transfer it to other cells and organisms.
- It is widely understood that individuals obtain some information from their parents through heredity, which allows the passing of anatomical and biochemical characteristics of the species from generation to generation.
- The transmission of hereditary information takes place in the nucleus of the cell.
- Certain structures within the nucleus, chromosomes, contain intermolecular complexes of basic proteins (histones) with acidic biopolymers called nucleic acids.
Nucleic acids
- Nucleic acids are condensation polymers of nucleotides, which in turn are the products of condensation of a nitrogenous base, a pentose sugar (ribose or deoxyribose), and phosphoric acid.
- In order to understand the functions of nucleic acids in living organisms we need to discuss first the structures and properties of their components, nitrogenous bases and nucleotides.
The structure of DNA
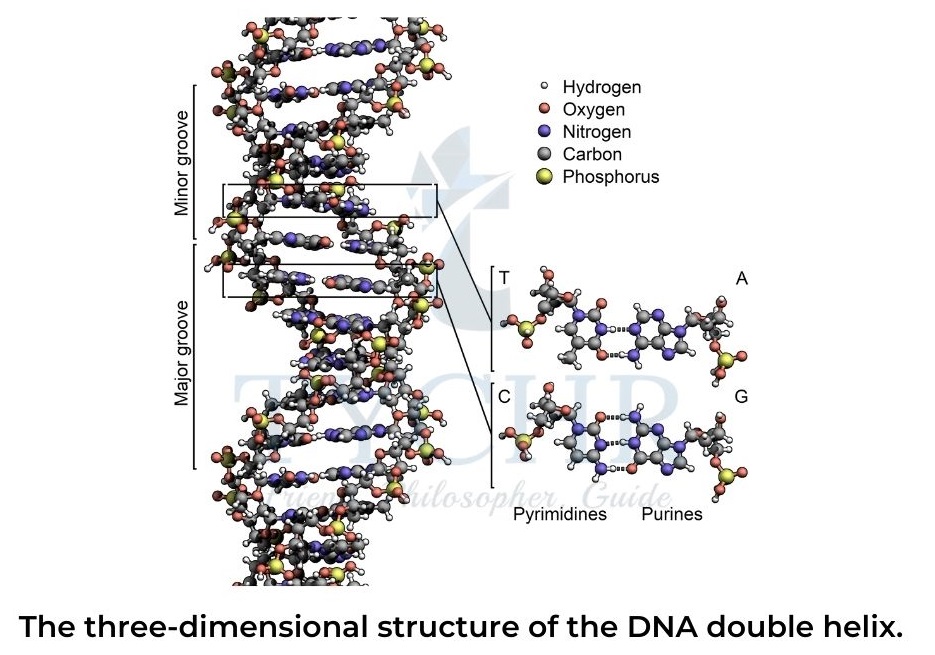
- DNA molecules consist of two polynucleotide strands in which each nitrogenous base from one strand forms a complementary pair with a nitrogenous base from another strand.
- Each pair contains one purine base (A or G) and one pyrimidine base (T or C, respectively).
Two hydrogen bonds in A=T base pairs and three hydrogen bonds in G≡C base pairs. - The double-helix shape of the DNA molecule stabilized by hydrogen bonds between complementary nitrogenous bases is known as its secondary structure.
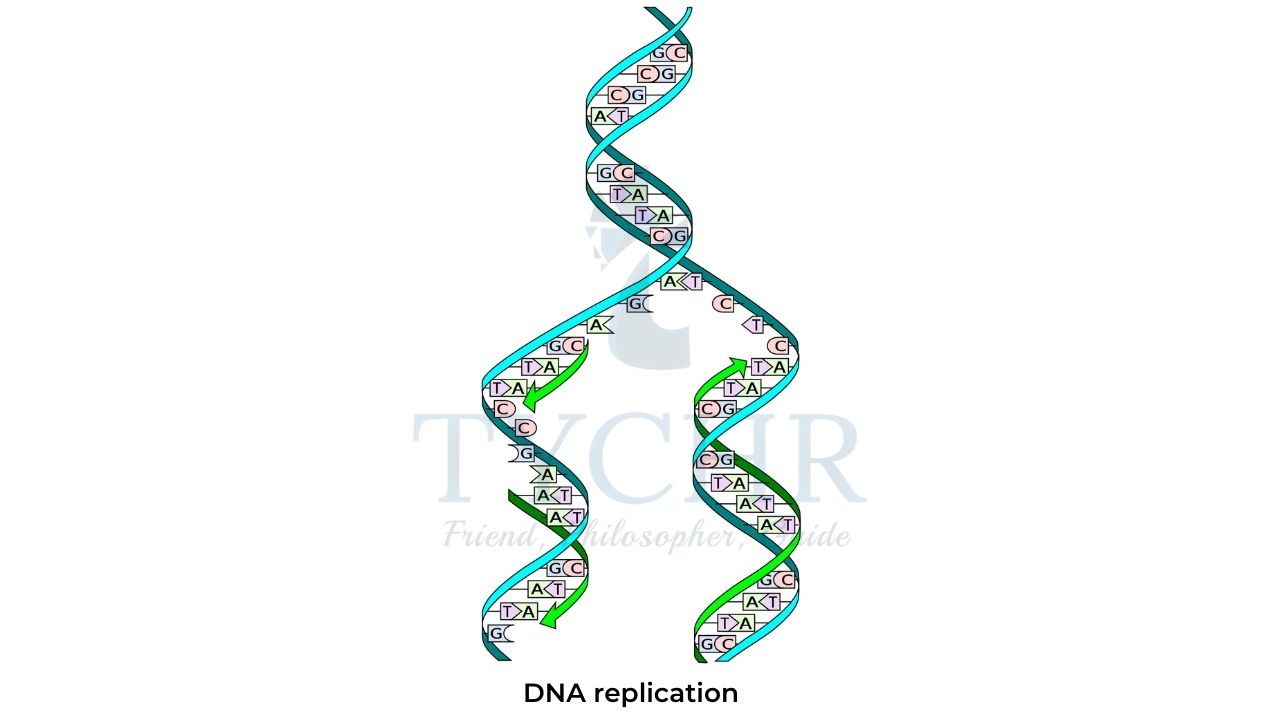
DNA replication
- The human body contains more than one trillion (1012) cells, most of which have very limited life spans and need to be replaced regularly. Because all cells of an individual organism contain identical DNA, there must be a mechanism by which exact copies of DNA molecules are created.
- This mechanism, known as DNA replication, is facilitated by several families of enzymes and includes three steps: initiation, elongation, and termination.
- The first group of enzymes, initiator proteins, separate the two DNA strands and create short polynucleotide fragments (primers) paired with the separated strands by complementary nitrogenous bases.
- Another group of enzymes, known as DNA polymerases, add more nucleotides to the primers using the existing DNA strands as templates.
- The resulting new polynucleotide chains are complementary to existing DNA strands and therefore produce two identical copies of the original DNA molecule.
- Finally, the replication process is terminated either by a certain sequence in the DNA or by the action of proteins that bind to specific DNA regions.
Transcription
- A mechanism similar to replication is used when an RNA molecule is created from a DNA template in a process called transcription.
- During transcription a DNA sequence is read by an RNA polymerase, which produces an RNA molecule complementary to an existing DNA strand.
- In contrast to the original DNA, the resulting RNA molecule contains ribose sugar (instead of deoxyribose in DNA) and uracil nitrogenous base (instead of thymine in DNA). In addition, RNA molecules usually exist as single polynucleotide strands with various three-dimensional configurations. The exact shape of an individual RNA molecule, known as its secondary structure, is determined by hydrogen bonds between complementary nitrogenous bases from different regions of the same strand.
- Each type of nucleic acid plays its own role in heredity.
- DNA resides in chromosomes, stores genetic information, and acts as a template from which this information is copied to RNA.
- The resulting RNA molecules transfer the genetic information from chromosomes to other regions of the cell and in turn are used as templates for protein synthesis.
- The latter process is known as translation and occurs in ribosomes, which are the largest and most complex molecular machines in cells.
- All living organisms use the same genetic code that allows ribosomes to translate three-nucleotide sequences (triplets, or codons) into sequences of amino acid residues in polypeptide chains.
Genetic engineering
- Detailed understanding of DNA structure and function led to the development of laboratory techniques for DNA manipulation.
- These techniques, known as genetic engineering, allow scientists to alter DNA sequences in the genes of living organisms, including the transfer of genetic material between different species.
- The resulting genetically modified organisms (GMOs) are used in scientific research, biotechnology, and agriculture. Various proteins, medicinal drugs, and other organic compounds are produced by genetically modified bacteria on an industrial scale.
- The most common GMOs, transgenic plants, possess many unique properties such as resistance to pests, viruses, and herbicides, tolerance to harsh environmental conditions, higher crop yields, and increased nutritional value.
- For example, golden rice is a species of Asian rice that was genetically modified to produce betacarotene, a precursor of vitamin A.
Pigments
Coloured compounds
- Most organic compounds are colourless because they do not absorb electromagnetic radiation in the visible range of the spectrum.
- Electron transitions in such compounds require relatively high energy, which corresponds to ultraviolet (UV) light and cannot be detected by the human eye.
- However, the presence of multi-centre chemical bonds and electron conjugation lowers the energy of electron transitions and therefore increases the wavelength of absorbed radiation.
- As a result, molecules with many delocalized electrons absorb visible light and appear coloured.
Carotenes
- Biological pigments are coloured compounds produced in living organisms.
- Their molecules usually have extensive systems of alternate single and double carbon–carbon bonds.
- The π-electron clouds of adjacent double bonds overlap and produce long chains of carbon– carbon bonds with delocalized electrons and an average bond order of 1.5.
- For example, in two members of group A vitamins, retinol and β-carotene, the electron conjugation involves 10 and 22 carbon atoms, respectively.
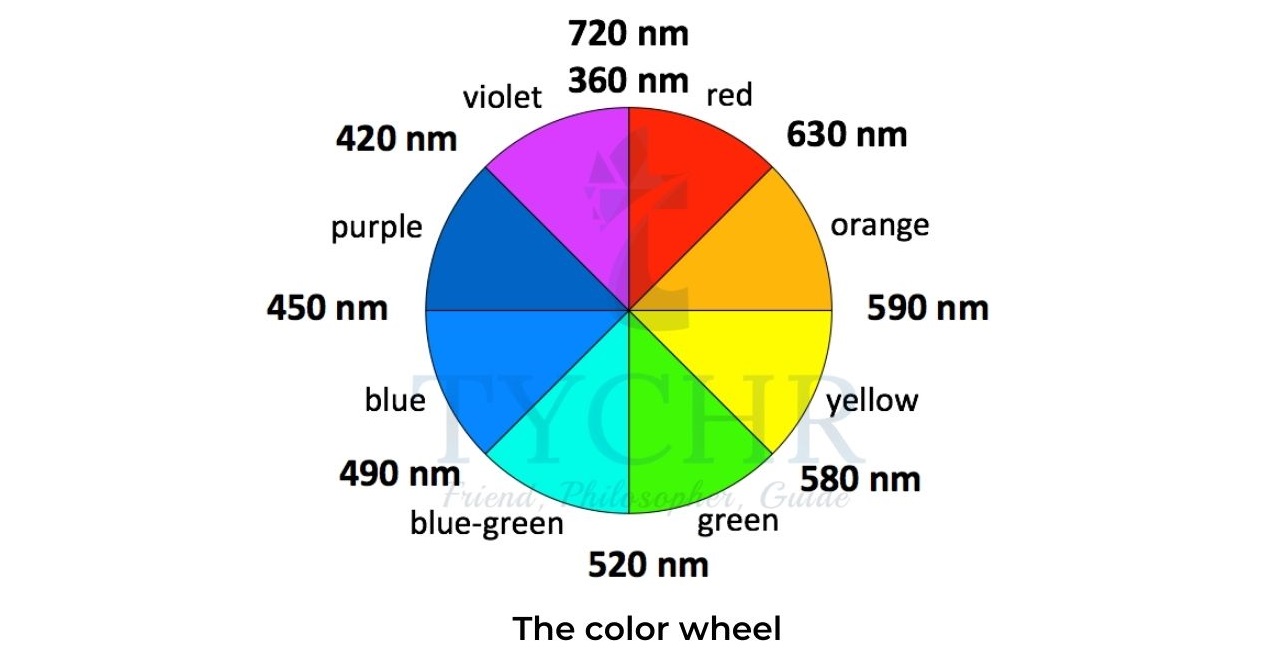
- The colour of a biological pigment depends on its molecular structure and on the number of delocalized electrons.
- Larger conjugation systems typically absorb light of lower energy, which corresponds to lower frequency and longer wavelength.
- If the wavelength at which the maximum of absorption occurs is known, the colour of the pigment can be predicted using the colour wheel.
Porphyrins
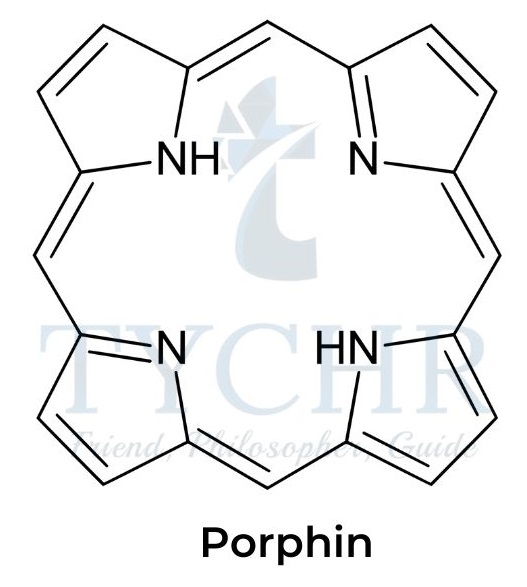
- Another important class of biological pigments, porphyrins, are complexes of metal ions with large cyclic ligands.
- The organic backbone of porphyrins, known as porphin, contains four nitrogen atoms in a highly conjugated aromatic heterocycle.
- Complexes of porphyrins with d-block elements absorb visible light due to electron transitions in the conjugate macrocyclic system and between d-orbitals of the metal ion.
- As a result, all proteins with prosthetic heme groups are brightly coloured.
- Myoglobin, the primary oxygenbinding protein in muscle tissue, contains an oxygen molecule bound to an iron(II) ion in heme, which is responsible for the characteristic red colour of raw meat.
- When meat is cooked the oxygen molecule in myoglobin is replaced with water and the oxidation state of iron changes from +2 to +3. As a result, cooked meat loses its original colour and becomes brown.
Cytochromes
- Molecular oxygen, which is supplied to cellular tissues by hemoglobin, is reduced to water during the final step of aerobic respiration.
- This step takes place in mitochondria and involves a group of enzymes collectively known as cytochromes. One of these enzymes, cytochrome c oxidase, is a large metalloprotein assembly containing four heme prosthetic groups and several ions of other metals including copper, magnesium, and zinc.
Chlorophyll
- Photosynthesis is a complex process that involves many pigments and proteins collectively known as photosystems.
- The light energy absorbed by some chlorophyll molecules is passed to other pigments in the reaction centre of the photosystem, where it is used to create a series of energy-rich molecular intermediates.
- These intermediates, known as the electron transport chain, undergo various redox reactions which ultimately lead to the oxidation of water to molecular oxygen and protons:
2H2O → O2 + 4H+ + 4e–
- In green plants, the protons produced by the above reaction are pumped through the chloroplast membrane and used for the synthesis of ATP in the same way as in mitochondria.
- The molecular oxygen produced by photosynthesis is released to the atmosphere as a part of the carbon– oxygen cycle.
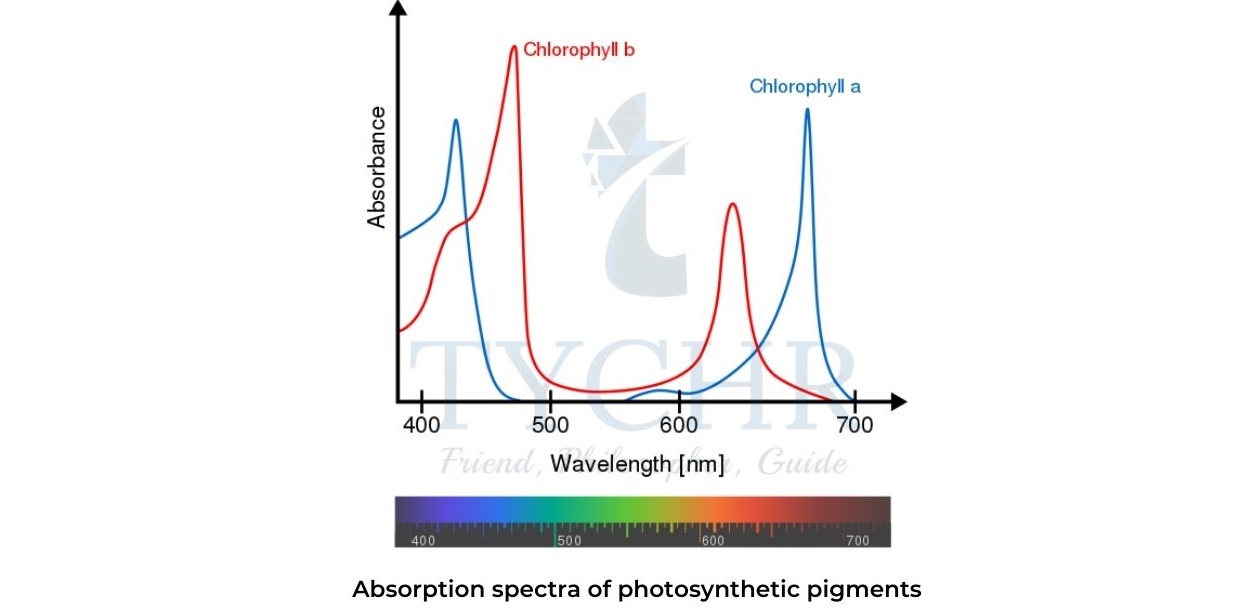
- Chlorophyll absorbs electromagnetic radiation in the blue and red regions of the visible spectrum.
- At the same time, green and neargreen portions of the spectrum are reflected or transmitted, which is responsible for the green colour of plant leaves and other chlorophyll containing tissues.
- Carotenes and some other pigments extend the absorption spectrum of chlorophyll and increase the efficiency of photosynthesis.
Stereochemistry in biomolecules
Stereoisomerism
- Most biochemical processes are stereospecific: they involve only molecules with certain three-dimensional configurations.
- Molecules that have the same sequence of atoms and chemical bonds but different arrangements of atoms in space are known as stereoisomers.
- Stereoisomers that cannot be transformed into one another without breaking a chemical bond are called configurational isomers and include two classes: cis-/transisomers and optical isomers.
- Both types of stereoisomerism play important roles in metabolic reactions, which are catalysed by enzymes with specific three dimensional structures.
- Most enzymes can bind only to those stereoisomers that fit into their active sites; other stereoisomers usually do not participate in normal metabolic processes.
- However, “wrong” stereoisomers can sometimes be recognized as substrates by different enzymes, act as non-competitive inhibitors, or accumulate in fatty tissues as xenobiotics.
- Such unwanted stereoisomers are often responsible for side effects of medical drugs and negative health effects of processed foods.
Fatty acids and triglycerides
- Naturally occurring unsaturated fatty acids have a cis-configuration of their double carbon–carbon bonds.
- The hydrocarbon chains in such molecules cannot adopt linear conformations, which prevents them from coming close to one another and reduces intermolecular forces.
- As a result cis-unsaturated fatty acids and their triglycerides, often referred to as cis-fats, are usually liquid at room temperature.
- Hydrogenation of vegetable oils is commonly used in the food industry to produce saturated fats with high melting points.
- The reaction of unsaturated triglycerides with hydrogen is similar to the hydrogenation of alkenes and takes place at high temperatures in the presence of a nickel or palladium catalyst.
- Hydrogenated vegetable oils are cheap and offer many benefits, including the absence of cholesterol, a controlled texture, spreadability, increased resistance to heat, and extended shelf life.
- However, the high temperature of the hydrogenation process leads to partial conversion of cis-fatty acid residues into their trans-isomers.
- If the hydrogenation is incomplete, the final product will contain trans-unsaturated triglycerides, known as trans-fats.
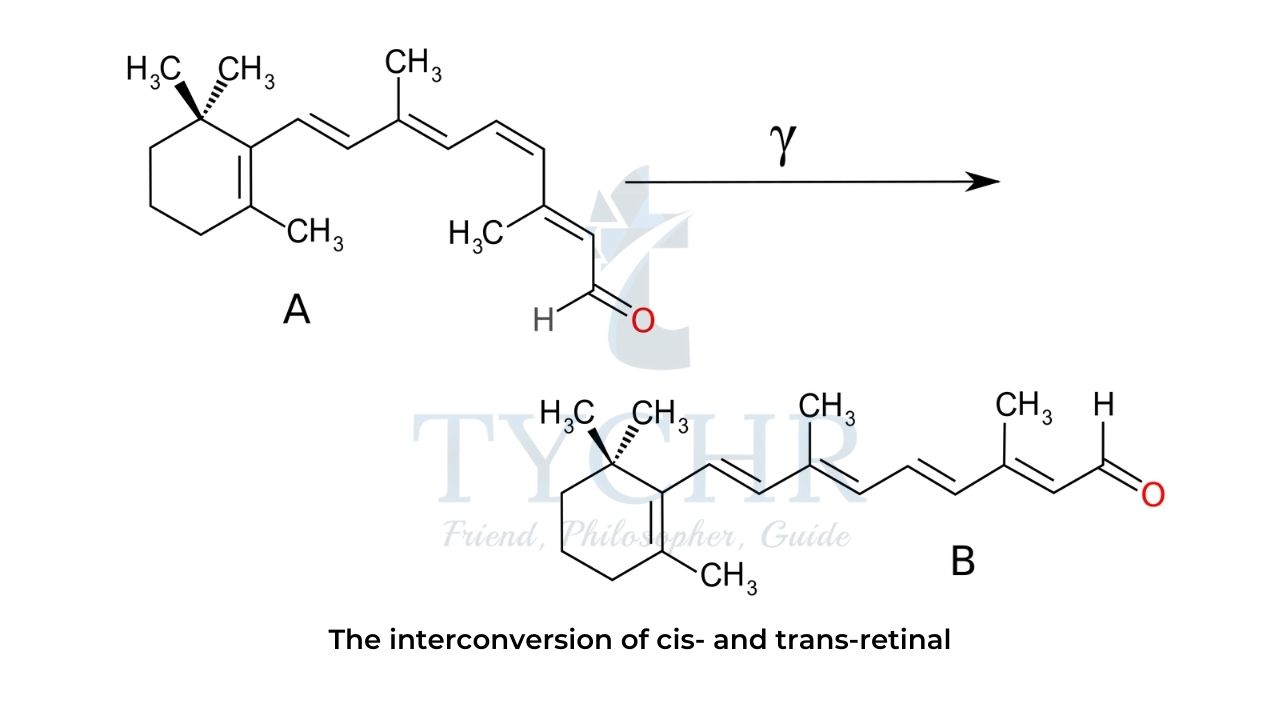
Retinal and vision chemistry
- The collective name “vitamin A” refers to a group of polyunsaturated compounds with diverse biological functions.
- One of these compounds, retinal, is a long-chain aldehyde involved in vision chemistry.
- In the photoreceptor cells retinal exists as two stereoisomers, cis-retinal and trans-retinal, which can be converted into one another by the action of visible light or enzymes.
- The aldehyde group of cis-retinal can reversibly bind to a lysine residue of the protein opsin, producing a light-sensitive pigment
- When rhodopsin absorbs a photon of visible light, the residue of cis retinal isomerizes into trans-retinal and the protein conformation changes, triggering a cell response that eventually sends an electrical signal to the nervous system.
- At the same time, trans-retinal detaches from opsin and undergoes a series of enzymatic transformations known as the visual cycle.
- At the end of the visual cycle, trans-retinal is converted back into cis-retinal, which reattaches to opsin and produces a new functional rhodopsin complex.
- In the human body, retinal can be synthesized only from other group A vitamins such as retinol or β-carotene.
- Insufficient production of retinal caused by a vitamin A deficiency leads to night blindness, which is a common medical condition in many developing countries.