option c energy Notes
Energy sources
Energy sources: quality and efficiency
- What makes a good energy source? It needs not only to contain a large quantity of potential energy but also for this potential energy to be released or converted, at a reasonable rate, to a useful form with minimal pollution and unwanted products.
- If the conversion is too fast a large quantity of the energy is dispersed, while if it is too slow it is not useful.
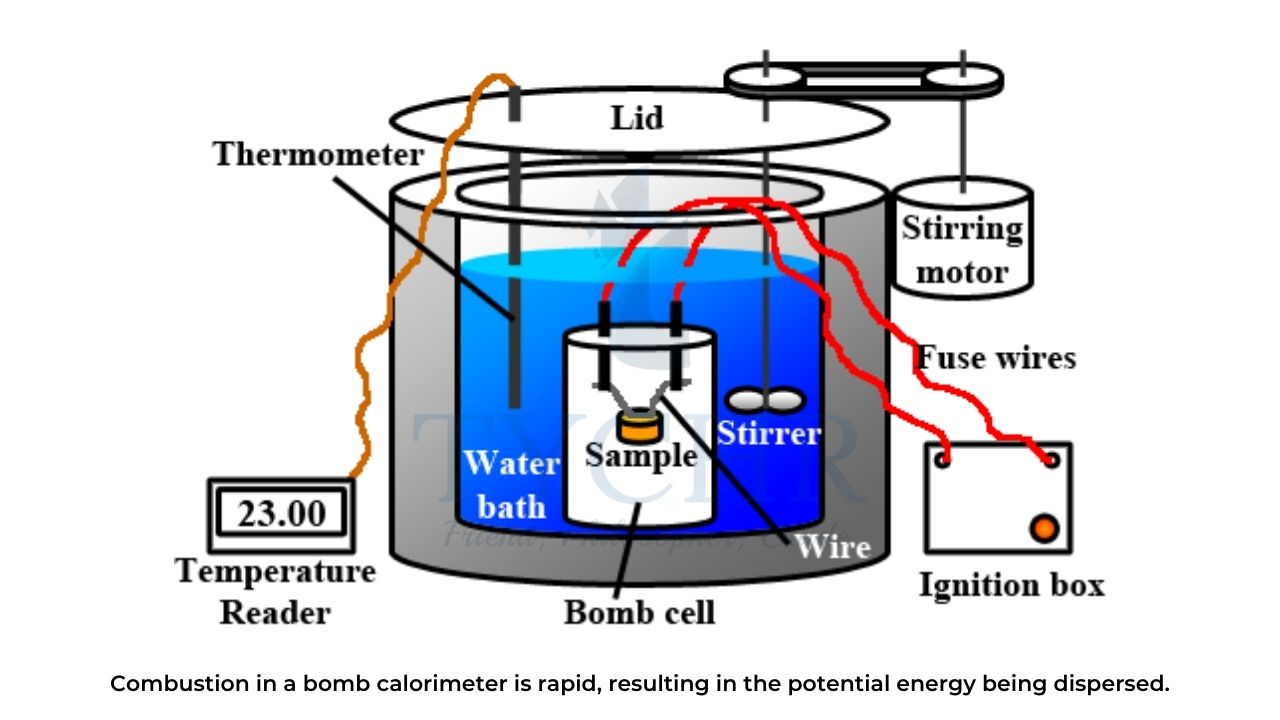
- The combustion of glucose is an exothermic reaction:
C6H12O6 (s) + 6O2 (g) → 6CO2 (g) + 6H2O(l) ΔH = -2803 kJ
- The same amount of energy is released when glucose is burnt in a bomb calorimeter as is released by its oxidation in the human body.
- The slower rate of oxidation in the body allows the energy to be converted to a useful form whereas the rapid oxidation of combustion disperses the energy too quickly, lowering its quality.
- The more the quality of energy is degraded, the less efficient the fuel is:
Efficiency of energy transfer = (𝐮𝐬𝐞𝐟𝐮𝐥 𝐨𝐮𝐭𝐩𝐮𝐭 𝐞𝐧𝐞𝐫𝐠𝐲/ 𝐭𝐨𝐭𝐚𝐥 𝐢𝐧𝐩𝐮𝐭 𝐞𝐧𝐞𝐫𝐠y) × 100%
- In a similar way the specific energy is the energy contained per unit mass of a fuel:
Specific energy = 𝐞𝐧𝐞𝐫𝐠𝐲 𝐫𝐞𝐥𝐞𝐚𝐬𝐞𝐝 𝐟𝐫𝐨𝐦 𝐟𝐮𝐞𝐥/𝐦𝐚𝐬𝐬 𝐨𝐟 𝐟𝐮𝐞𝐥 𝐜𝐨𝐧𝐬𝐮𝐦𝐞𝐝
Renewable energy resources
- Some renewable or “green” energy resources include solar energy, wind energy, biomass,
water (such as tides, currents, and waves), geothermal energy, and fuel cells. - Geothermal energy is one of the more widely used commercial forms of renewable energy resources.
- Although it has an efficiency of only about 23%, as with all energy resources, it is important to consider not only the efficiency of conversion but also the cost per kilowatt-hour.
Fossil fuels
Storing energy from photosynthesis
- The harnessing of energy from the sun by photosynthesis enabled the emergence of large organisms.
- As these organisms died and decayed, the strong C–C and C–H bonds in them remained intact and these are the source of our main energy supply today.
Fossil fuels store reduced carbon
- Many fossil fuels contain saturated alkanes.
- During fossil fuel formation carbon atoms become more and more saturated with hydrogen and have fewer bonds to nitrogen, sulphur, and/or oxygen than existed in the living form.
- The carbon–hydrogen bond is relatively stable and stronger than single bonds between carbon and oxygen, sulphur, or nitrogen.
Crude oil: Fractionating and cracking
- There are three main fossil fuels: coal, gas, and crude oil. Crude oil or petroleum is by far the most important yet this “black gold” is difficult to use in its natural form.
- It contains a vast mixture of hydrocarbons of varying chain lengths. Long-chain hydrocarbons have stronger van der Waals’ intermolecular forces between them than do the shorter chains, so their differing boiling points can be used to separate crude oil into “fractions” of various chain lengths. At oil refineries the various fractions are separated by distillation.
- The more volatile shorter-chain hydrocarbons make better fuels and they burn with a cleaner flame.
- However there is a much larger percentage of long-chain hydrocarbons in crude oil than short-chain ones.
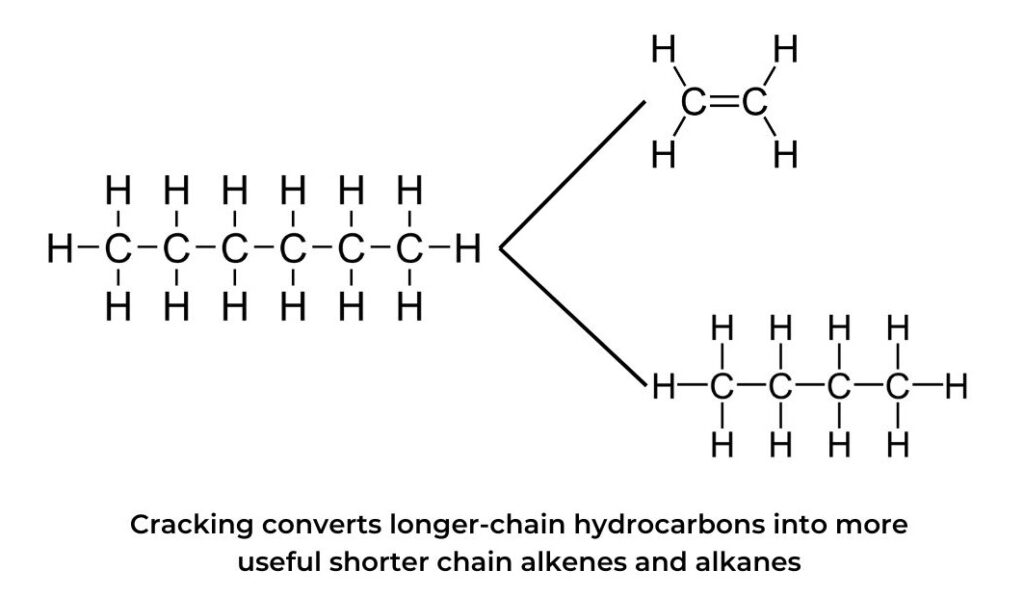
- In order to obtain more of the desired short-chain fuels a process called cracking is employed. Fractions such as naphtha that contain longer-chain hydrocarbons are heated over a catalyst where they are “cracked” into smaller hydrocarbons including alkenes such as ethene and the more usable alkanes such as the octanes used in petrol.
Fuels and octane rating
- When fuels are burned in automobile engines they are first compressed and then ignited with a spark. Some hydrocarbons have a higher tendency than others to “auto-ignite” during this compression stage.
- This produces an effect known as “knocking” which can severely damage engines.
- The fuel’s octane rating indicates its resistance to auto-ignition.The length and degree of branching of the hydrocarbon chain have the following effect on the octane rating.
- Octane rating increases with branching. 2,2,4- trimethylpentane has a higher octane rating than octane. They both contain 8 carbon atoms but the more highly branched 2,2,4- trimethylpentane is more resistant to auto-ignition.
- Octane rating decreases with the length of the carbon chain. Hexane has a higher octane rating than heptane.
- The octane rating of aromatics is higher than that of straight-chain or branched-chain alkanes with the same number of carbons. Benzene with 6 carbons has a higher octane rating than either hexane or 2-methylpentane.
Catalytic reforming
- Catalytic reforming is used to convert low-octane numbered alkanes such as heptane or octane into higher-octane numbered isomers such as methylbenzene or 2,2,4- trimethylpentane.
- The straight-chain alkanes are isomerized by heating with a platinum catalyst. Their chains break apart and reform, increasing the proportion of branched alkanes.
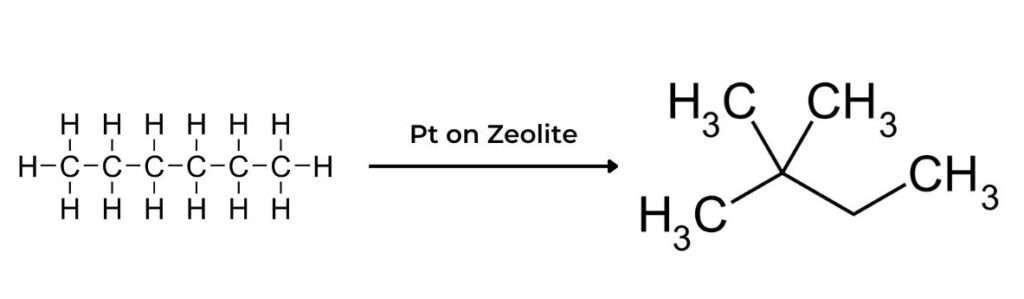
- Using a platinum catalyst with aluminium oxide, or other metal catalyst, reforms and dehydrogenates the alkane into an aromatic compound. For example, heptane can be converted into methylbenzene and hydrogen gas:
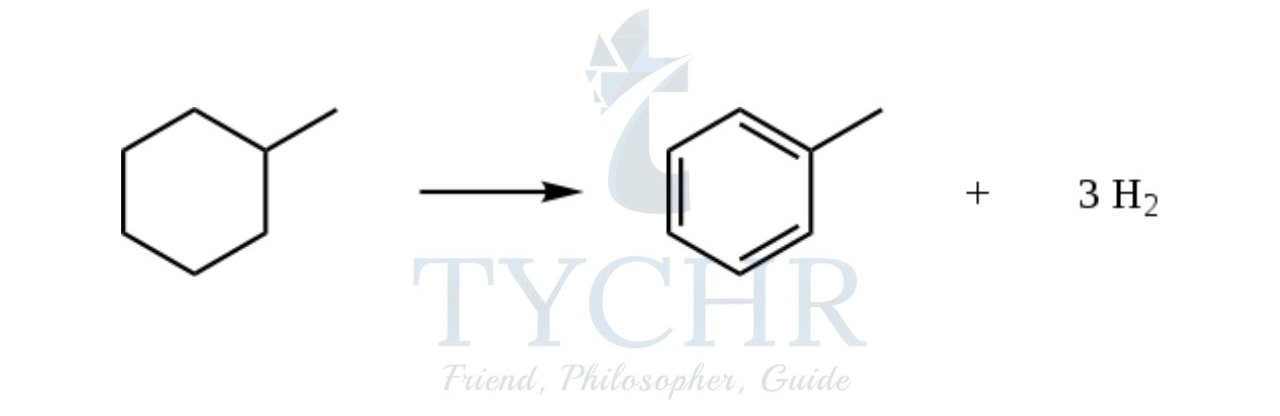
- Reforming is the summative effect of several reactions such as cracking, unifying, polymerizing, and isomerizing occurring simultaneously.
- It is used to produce high-octane alkanes or other useful aromatics such as methylbenzene. At an oil refinery crude oil is treated by a combination of distillation, cracking, and reforming to produce the valuable products that drive our society today.
Coal gasification
- Coal is a more abundant fossil fuel than crude oil, and can be converted to other more useful forms that are cheaper than crude oil.
- One method is coal gasification in which synthesis gas, also called coal gas or syngas, is produced by reacting coal with oxygen and steam in a gasifier to create hydrocarbons.
- Inside the gasifier the oxygen reaching the coal is limited so that combustion will not occur.
- This is an example of carbon capture and storage (CCS) which involves capturing carbon dioxide from large industrial processes, compressing it, and transporting it to be injected deep into rock formations at selected safe sites.
- This reduces the amount of carbon dioxide entering the atmosphere.
- Gasification produces other products including slag which is used in roofing materials or for road construction, methanol, and nitrogen-based compounds for fertilizers.
“Green”fuels and the carbon footprint
- The production of energy by burning fuels produces carbon dioxide.
- The carbon footprint of a reaction is a measure of the net quantity of carbon dioxide produced by the process.
- Even though biofuels may cost more to produce, their carbon footprint is less because carbon dioxide is absorbed by photosynthesis while the fuel is growing.
Nuclear fusion and fission
Hydrogen fusion
- The fusion of hydrogen nuclei is the source of the sun’s energy. This fusion reaction releases
much more energy than the fission of U-235 or Pu-239, the fuels used in nuclear reactors. - In the sun hydrogen nuclei or protons combine to form the isotope deuterium 2H, which then further combines to form helium nuclei.
- We will recall that a helium nucleus is composed of 2 protons and 2 neutrons.
- A helium nucleus, on the other hand, has a mass that is lower than the sum of the masses of two protons and two neutrons. The term for this is the mass defect.
Nuclear processes: Fusion and fission
- Many different chain reaction mechanisms can occur to produce the helium nucleus, and most of them occur in the sun and stars. One of the proposed mechanisms for producing energy by controlled nuclear fusion here on Earth is the fusion of deuterium (a hydrogen isotope with 1 proton and 1 neutron) with tritium (a hydrogen isotope with 2 neutrons):
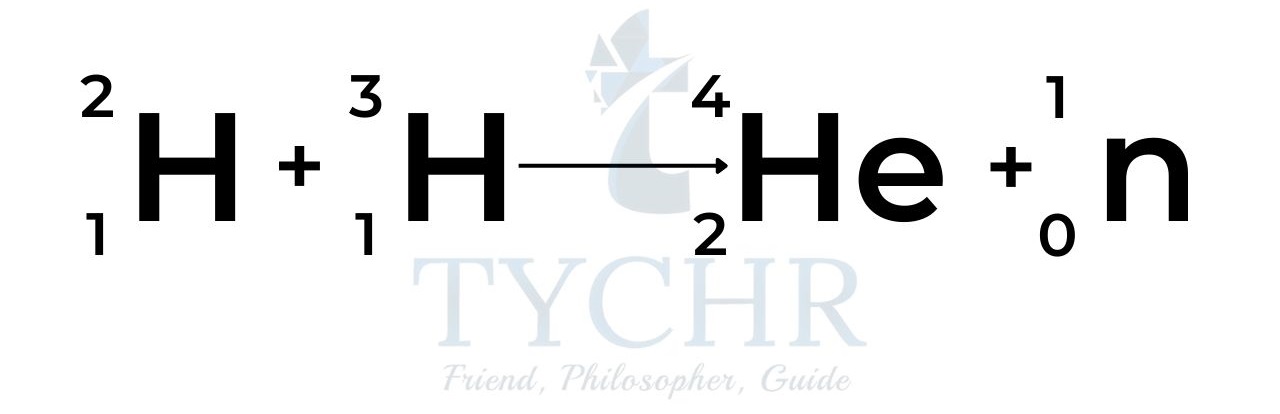
- Iron has the most stable nuclear configuration. By fusing lighter elements to form larger ones the binding energy increases and the mass defect is converted to energy.
- On the other hand, the heavier transuranium elements (those with atomic number greater than 92) can undergo splitting or nuclear fission to form two lighter nuclei.
- As the sum of the binding energies of the two lighter elements is greater than the binding energy of a uranium-235 isotope, there is a mass defect which is converted directly to energy.
- The chain reaction is sustainable provided one neutron from the fission of U-235 strikes another U-235 atom, causing further fission to occur.
- The amount of material needed for the reaction to remain sustainable is the critical mass.
- At the point where the number of neutrons produced in one generation is equal to the number of neutrons produced in the next generation the reactor is referred to as critical.
Types of subatomic particle
- The conversion of one element to another by capture or emission of a particle is referred to as transmutation.
Particle | Symbol | Description and hazard |
| alpha particle | α or 42He | A helium nucleus consisting of 2 protons and 2 neutrons. It is the most massive particle involved in radioactive reactions and can travel only a few centimetres in air. Limited hazard unless inhaled or ingested. |
| beta particle | β or 0-1e | A high speed electron with negligible mass and a charge of -1. Beta particles are a product of nuclear decay. They have a range of a few metres and have enough energy to cause burns to the skin. |
| gamma ray | γ | High frequency, short wavelength electromagnetic waves. Due to their short wavelength they have a high penetrating ability. They can cause cancer but under controlled conditions are used in medicine for treatment, imaging, and sterilization. |
| neutron | 10n | Uncharged nuclear particle with a mass of 1 atomic mass unit. May be emitted in fission and fusion reactions. They have a high penetrating ability and can be damaging to biological material |
| positron | 0 +1β+ | The antiparticle of an electron; a positively charged beta particle. |
| proton | 1 1p or 1 1H | Nuclear particle that has a mass of 1 atomic mass unit and a charge of +1 atomic mass unit. |
Table 1: Subatomic particles involved in fusion and fission reactions
The half-life of a nuclear process
- Some heavier atoms are radioactive – they undergo spontaneous decay to produce daughter products, releasing alpha, beta, and/or gamma radiation in the process.
- Radioactive decay is a first order reaction, meaning that it has a constant half life.
- The half-life (t1/2) refers to the time it takes for one half of the number of atoms in a sample to decay.
- the following equation allows us to calculate the half-life if we know how much material we started with (N0), how much remains (N), and the time interval (t):
t1/2 = t((ln2)/ln(𝑁𝑜/𝑁))
Nuclear energy
- Nuclear energy allows us to obtain large quantities of energy from small quantities of matter, making it a very important industry.
- Using the Einstein mass–energy equivalence relationship, the mass difference between the reactants and products can be used to calculate the energy produced in a nuclear reaction.
- The mass defect is the difference between the mass of the nucleus and the sum of the masses of its nucleons (protons and neutrons), and its relationship to the nuclear binding energy.
- The nuclear binding energy (ΔE) is the energy required to separate a nucleus into protons and neutrons.
Radioactive decay
- Radioactive decay is kinetically a first order process. The time it takes for half of the sample to decay is the half-life t1/2.
- A quantity called the decay constant, λ, is related to the half-life by the following equation:
λ = ln2/(t1/2)
- The decay constant, λ, is the first order rate constant for the decay.
Solar energy
Photosynthesis: Harnessing solar energy by chlorophyll
- Sunlight is absorbed in chloroplasts by the chemical chlorophyll.
- Visible light can be absorbed by molecules that have a conjugated structure with an extended system of alternating single and multiple bonds. These alternating bonds in chlorophyll can absorb light energy.
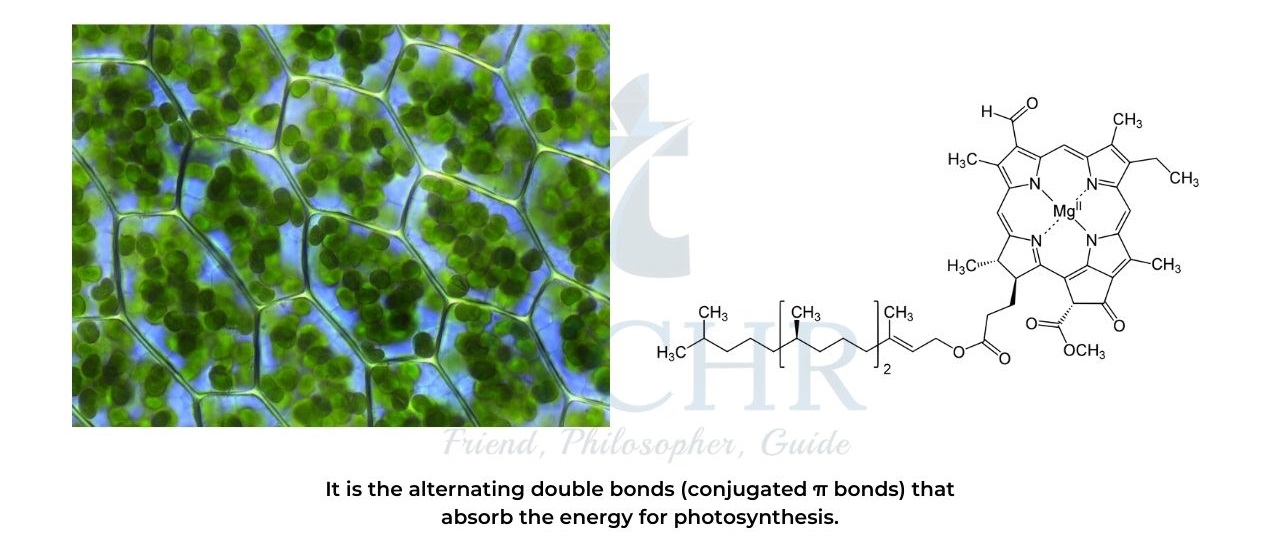
- Pigments in plants are coloured due to conjugated double bond systems.
- If a certain pigment absorbs red and green, or yellow, light as a result of its extended conjugation, then blue or purple light will be reflected.
- Violets are blue (or violet) because of anthocyanin pigment in the flower.
Biofuels
- The conversion of carbon dioxide to carbohydrates using solar energy by photosynthesis produces our food and fuels. Biofuels such as ethanol are obtained from corn sugar or glucose by fermentation:
C6H12O6 → 2C2H5OH + 2CO2
- The carbon dioxide produced in the fermentation process is balanced by carbon dioxide taken in for photosynthesis while the plant is growing, so the fuel can be considered carbon neutral; its use instead of petrol also conserves fossil fuels.
- Biodiesel is another sustainable fuel that can be grown and used as a substitute for diesel. It is produced from vegetable oils, which can release similar amounts of energy to diesel when burnt.
- However, because they are highly viscous they are unable to flow easily and can clog fuel injectors.
- Large forces between molecules are implied by a high viscosity; These oils frequently undergo incomplete combustion, which further damages engines because they do not readily vaporize.
Some advantages and disadvantages of biodiesel are summarized
Advantages | Disadvantages |
| High flash point (less flammable than normal diesel) | More viscous than diesel, even when converted to methyl esters – requires pre-warming. |
| Lower carbon footprint – amount of CO2 produced is the same, but CO2 was consumed in growing the plants. For petroleum cars CO2 is introduced into the atmosphere that wasn’t there before. | Slightly lower energy content than petroleum-based diesel. Uses agricultural resources resulting in increased food prices on a global scale |
| More easily biodegradable in the event of an oil spill. Sulfur free so produces no SO2 emissions. | The production of biodiesel from raw materials is more costly than the production of diesel from fossil fuels. |
| Sustainable – the raw materials can be grown using solar energy as the source. | Biofuels may contain more nitrogen than fossil fuels and thus release more nitrogen oxides, NO and NO2 , when burned. |
| A good solvent – cleans engines. | Dirt cleaned from engines tends to clog fuel filters and cause cars to stall. It can also dissolve paint and protective coatings. |
Table 2: Some advantages and disadvantages of biodiesel compared with diesel
Environmental impact: global warming
The natural greenhouse effect
- The radiation in sunlight has a range of wavelengths.
- The highest frequencies are absorbed by the upper atmosphere, allowing some UV, visible, and longer wavelengths to reach the surface where they are absorbed.
- The waves re-emitted from the surface are longer wavelength infrared (IR).
- These waves interact with carbon dioxide, methane, and water vapour, the main greenhouse gases, which capture this energy so that it remains trapped in the Earth’s atmosphere.
- This natural effect of the atmosphere is similar to a greenhouse, hence the term ‘greenhouse effect’.
Natural sources of greenhouse gases
- The vast majority of atmospheric water vapour is of natural origin and accounts for 95% of all greenhouse gases.
- There is a natural balance between liquid water on the Earth’s surface and vapour in the atmosphere.
- As the Earth warms up, more surface water evaporates and this increases the atmospheric water vapour concentration.
- The atmosphere then absorbs more IR radiation and causes increased warming. However, much of the water vapour condenses into clouds which block sunlight, causing global dimming and cooling the planet.
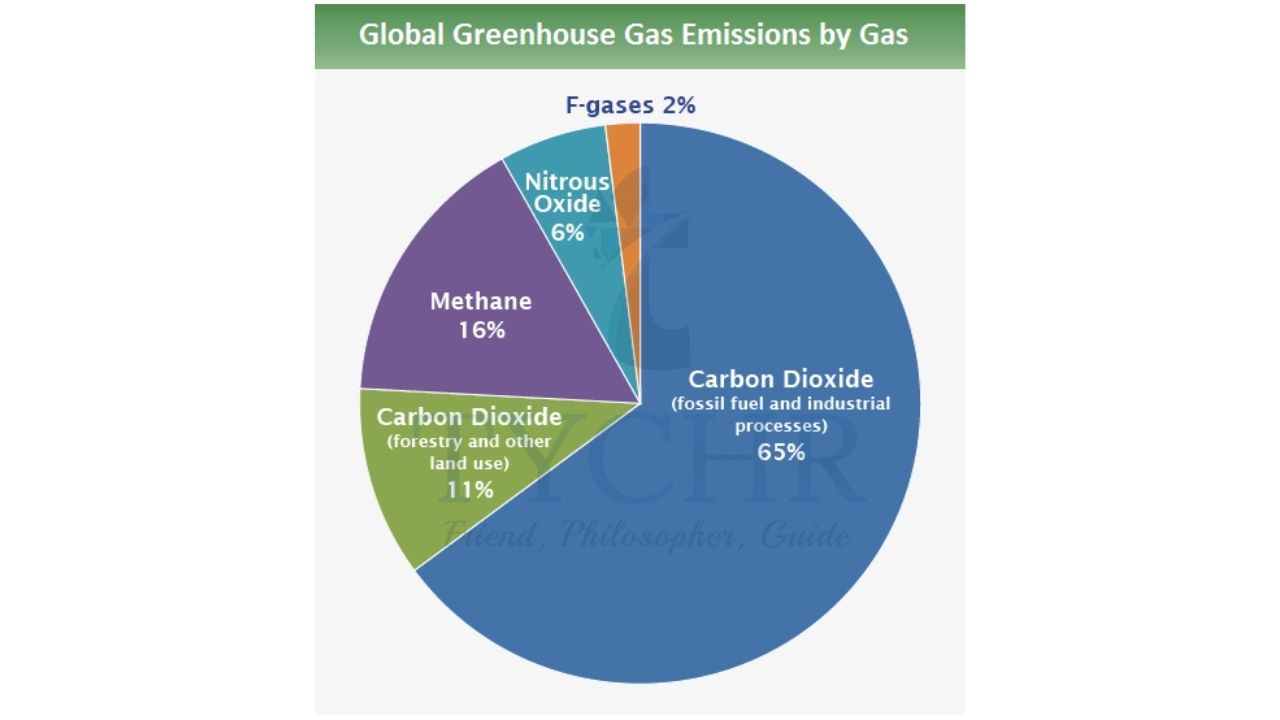
Greenhouse gas emission from human activities
- The main sources of anthropogenic greenhouse gases (those arising from human activity) are listed below.
- Burning coal, oil, and natural gas for energy production accounts for nearly 50% of anthropogenic greenhouse gases. The carbon dioxide entering the atmosphere as a combustion product comes from hydrocarbons that were previously stored underground, so this increases absolute levels of the gas in the atmosphere. Water vapour is also a combustion product but the increase in water vapour is small compared with the increase in carbon dioxide levels.
- Industrial gases from factories introduce not only carbon dioxide but also new greenhouse gases such as nitrogen oxides (NOx ) accounting for approximately 25% of human greenhouse gas production. Some of these gases, such as chlorofluorocarbons (CFCs), do not occur naturally.
- Agriculture and deforestation account for the remaining 25%, with each contributing nearly equally. Agriculture increases methane concentrations from ruminant animals such as sheep and cows who generate methane in their digestive systems. Deforestation increases carbon dioxide because with fewer trees, less carbon dioxide is absorbed from the atmosphere and used in photosynthesis.
Carbon sinks: The role of the oceans
- Of all the carbon dioxide gas released to the atmosphere by human activity, approximately half has remained in the atmosphere. The rest is removed to carbon sinks such as the oceans.
- About 30% of anthropogenic CO2 is absorbed by the oceans.
- Carbon dioxide itself is not very soluble, with the heterogeneous exchange between carbon dioxide gas and aqueous carbon dioxide occurring at the ocean’s surface.
Measures to reduce greenhouse gas emissions
- International government agencies have begun to cooperate both to reduce the emission of greenhouse gases and to stop deforestation so that more CO2 can be removed from the atmosphere for photosynthesis.
Industry and energy production
- Carbon capture and storage (CCS) is the process of capturing waste carbon dioxide from where it is produced, such as fossil fuel power plants, transporting it to a storage site, and storing it where it will not enter the atmosphere, such as in an underground geological formation.
Agriculture and deforestation
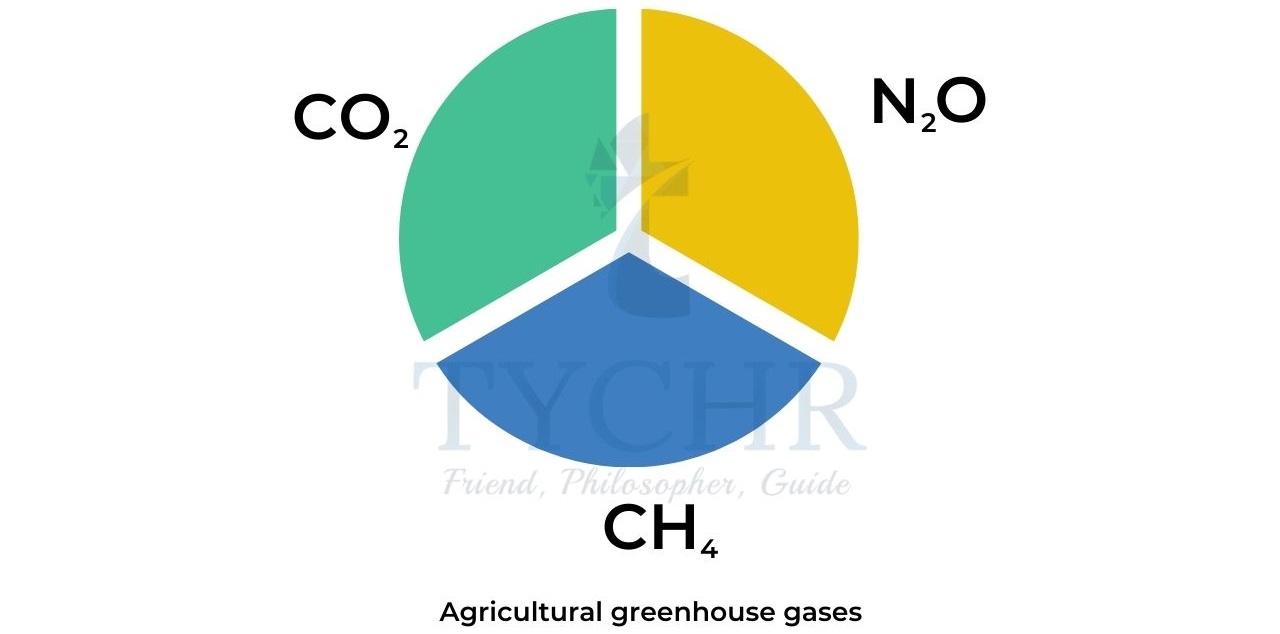
- Methane, CH4 and nitrous oxide, N2O are the main greenhouse gases produced in agriculture. Although these two gases are produced in smaller quantities than carbon dioxide they still have a pronounced effect.
- Methane is 25 times as powerful a greenhouse gas as carbon dioxide while nitrous oxide has over 300 times the impact.
- Taking this into consideration rather than simply the quantities of gases produced, the livestock (dairy and beef) industry produces a large percentage of agricultural greenhouse gases by enteric fermentation, anaerobic decomposition of organic matter, and fertilizer use.
- Deforestation to create agricultural land should be carbon neutral as crops rather than trees are being grown, but this is not the case if use of fertilizers is increased.
- The use of urban space to grow crops could subsidise local communities and reduce transport costs.
Global dimming
- Smoke, dust particles, and clouds reflect sunlight back to space, causing global dimming which cools the Earth’s surface.
- Particulate matter such as soot and ash can further change the properties of clouds.
- Small droplets of water start to collect (nucleate) on tiny particulates and intermolecular forces between pollutant particles and water droplets result in the droplets collecting to form clouds.
- So by the process of global dimming, fossil fuel pollutants reduce as well as increase global warming. However, global dimming has harmful effects such as:
- Certain types of pollutant can cause acid rain.
- Global dimming decreases the rate of evaporation of water, which can reduce monsoon rains and lead to a reduction in crop yields in areas of the world where they are most needed.
- Pollution causes local health problems such as asthma.
The effects of global warming on climate change
- Observed measures of climate change include melting permafrost, less radiation reaching the Earth’s surface, more devastating storms occurring, temperatures becoming more extreme (both hotter and colder), and record levels of rainfall and droughts.
- There appears ample evidence that anthropogenic greenhouse gas emission is raising global temperatures and is linked to global dimming.
- Radical changes in climate could put pressure on food and water resources for the growing worldwide population.
Electrochemistry, rechargeable batteries, and fuel cells
Primary and secondary cells
- A battery is a series of portable electrochemical cells.
- In a primary electrochemical cell the materials are consumed and the reaction is not reversible.
- Either the anode, electrolyte, or both need to be replaced or the battery is thrown away, which is usually cheaper. Typically the anode (negative electrode) is oxidized and can no longer be used.
- In a secondary cell or rechargeable battery, the chemical reactions that generate electricity can be reversed by applying an electric current to them.
- Secondary cells can deliver stronger current demands than primary cells. Secondary cells have a higher rate of self discharge than do primary cells.
- When you purchase a replacement battery for a phone, for example, you would need to charge it before use as it will have self discharged and so will be only partially charged.
Secondary cells: Lead–acid batteries
- Rechargeable batteries are used in cars, for energy storage in the electric grid (such as to store energy generated from solar cells), in motorized electric vehicles (hybrid cars, golf carts, etc.), as emergency back-up, and for many other uses.
- The typical lead–acid battery in a car is recharged while driving.
- Electrical energy is used to create ignition and then some of the energy from combustion is used to reverse the chemical reaction in the battery, keeping it charged ready for next time.
- The continual charging of a battery tends to produce some overvoltage which produces hydrogen and oxygen from water.
- This is why nonsealed car batteries occasionally need to be topped up with distilled water.
Secondary cells: Lithium-ion batteries
- Lithium-ion rechargeable batteries use lithium atoms absorbed into a lattice of graphite electrodes rather than pure lithium metal for the anode.
- The cathode is a lithium cobalt oxide complex, LiCoO2.
- The lithium atoms are oxidized to lithium ions during discharge.
- As lithium has the highest oxidation potential (most negative reduction potential) and is lightweight, it is an ideal material for lightweight batteries.
- The lithium-ion battery has a very high charge specific density compared with other rechargeable batteries such as lead–acid or nickel–cadmium batteries.
- Lithium-ion batteries store and deliver 6 times as much energy per kilogram as a lead–acid battery. Some other advantages are:
- They hold charge better than either nickel–cadmium or lead–acid batteries
- They can withstand many recharge cycles
- They contain no heavy metals so used batteries are considered safe for disposal in normal landfill sites.
The voltage of a cell
- The voltage of a battery, whether primary or secondary, depends on the nature of the anode and cathode.
- The further apart the standard electrode potentials of the oxidizing and reducing materials, the more voltage per cell is available.
- Placing cells in series provides an increased voltage. Lead–acid car batteries use many such cells and usually provide 12 V.
- The total number of electrons moving along with the energy given to them by the cell gives a measure of how much work can be done by the current.
- This in turn depends on the nature and quantity of the materials (the mass and surface area of the electrodes) as well as the specific energy density.
- It is the electrons moving in the external circuit that provide us with useful energy but each electrochemical cell also has to move cations and anions inside the cell.
- A battery’s internal resistance depends on the ion mobility, the electrolyte conductivity and the electrode surface area.
Hydrogen fuel cells
The PEM fuel cell
- A fuel cell is an electrochemical device that converts the chemical potential energy in a fuel into electrical energy.
- In the hydrogen fuel cell the fuel is hydrogen, which is oxidized by oxygen and produces water. There is therefore no pollution and fuel cells are very efficient.
- The key components of a fuel cell are:
- the electrolyte or separator which prevents components from mixing – the proton exchange membrane (PEM) is a polymer which allows H+ ions to diffuse through but not electrons or molecules (acts as a salt bridge).
- the oxidizing and reducing electrodes which are catalysts that allow the chemical reactions to occur
- the bipolar plate which collects the current and builds up the voltage in the cell.
Alkali fuel cells
- Alkaline fuel cells were used as early as the Apollo missions to provide electricity and drinking water.
- The electrolyte in these cells was a solution of potassium hydroxide, providing a source of hydroxide ions.
- As the OH– ions migrate towards the anode they react with H+ ions producing water.
Microbial fuel cell
- A microbial fuel cell converts chemical energy available from a substrate into electricity by anaerobic oxidation carried out by microorganisms.
- The aerobic oxidation of glucose produces carbon dioxide and water. However, anaerobic oxidation produces H+ ions and electrons.
- These electrons can be harnessed at an anode and the H+ ions permitted to diffuse through a PEM where they reduce oxygen, forming water.
Photovoltaic and dye-sensitized solar cells (DSSC)
Conjugated systems
- Conjugation is the interaction of alternating double bonds, for example in organic molecules, to produce a delocalized array of pi electrons over all the atoms.
- Molecules with conjugated bonds can absorb visible light, while longer conjugated systems absorb light of longer wavelength.
- All the carbon atoms involved in such systems have sp2 hybridization: the π-electron clouds of adjacent double bonds partly overlap with one another and form a large cloud of delocalized electrons.
- This type of multi-centre chemical bonding known as electron conjugation is similar to the electron delocalization seen in benzene and produces a chain of carbon–carbon bonds with a bond order of 1.5.
Silicon semiconductor photovoltaic cells
- Semiconductors have electrical conductivity midway between that of conductors and insulators.
- The conductivity of a semiconductor increases with temperature, in contrast to that of conductors. Conductors are typically metals with low ionization energies and therefore freely moving electrons.
- When heated, lattice movement increases which interferes with conduction. However, semiconductors are relatively poor conductors of electricity due to their higher ionization energies.
- Photovoltaic cells made of semiconductors can absorb photons of light resulting in electrons being knocked free from atoms and creating a potential difference. Semiconductor materials for such cells are often pure group 14 elements such as silicon or germanium.
- The conductivity of the semiconductor can be increased by “doping” it with small impurities of group 15 elements, such as phosphorus to create an n-type semiconductor, or a group 13 element such as boron to create a p-type semiconductor.
- In summary: The photovoltaic cell absorbs photons in a semiconducting material, which causes some valence electrons to be removed, resulting in some ionization in the cell.
- A charge separation occurs in the semiconductor which allows for a one-way flow of electrons.
- The cell can be linked to an external circuit where the low of electrons provides electrical power.
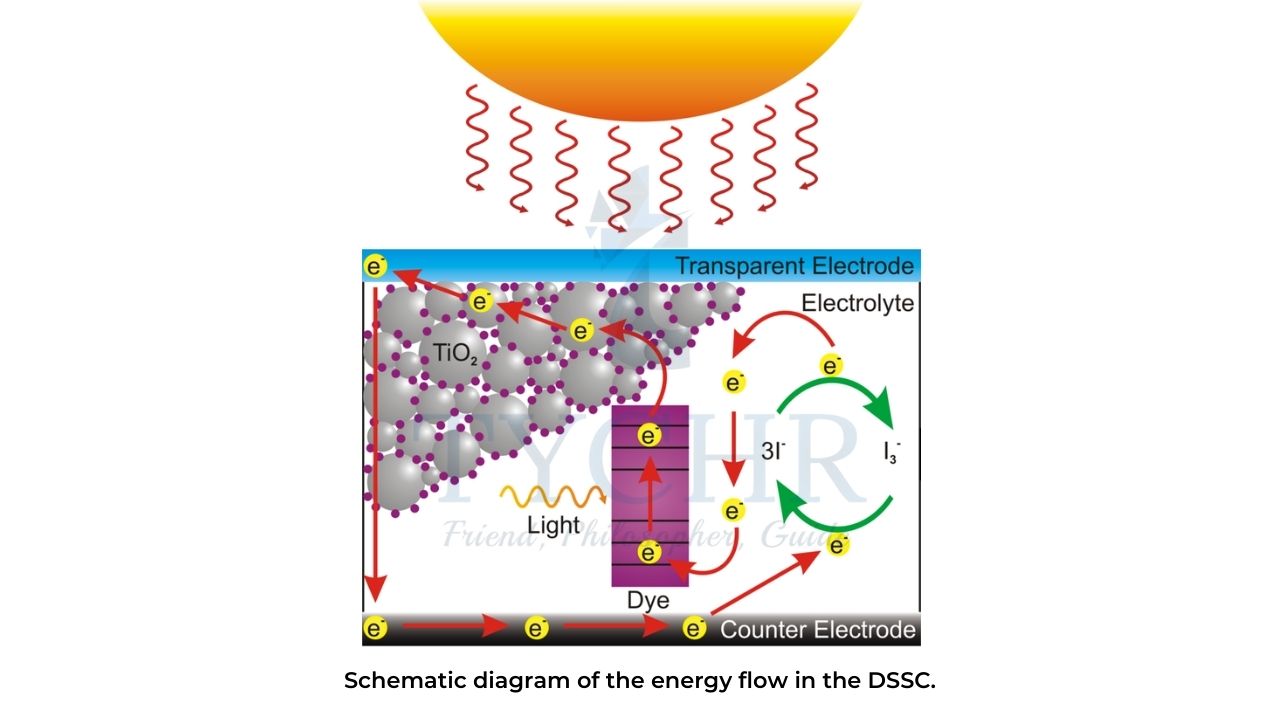
Dye-sensitized solar cells (DSSC)
- Once a dye molecule has emitted its excited electron it needs to gain another electron.
- To achieve this, dye-coated TiO2 nanoparticles are immersed in a solution of iodide ions, I–.
- The iodide ions release electrons to the dye on the TiO2 layer, becoming oxidized to tri-iodide I3–.
- This can accept electrons at the cathode, being reduced back to I–.
- An outline of the process is shown in figure.