metabolism, cell respiration and photosynthesis Notes
Metabolism, cell respiration, and photosynthesis
Approaching the topic:
- Understanding the concepts
- Application of these concepts
Metabolism
Definition and mechanism
- Metabolism is the sum of all the chemical reactions taking place inside a living organism, most of which are enzyme-mediated reactions.
- These enzymatic reactions when converting large molecules into smaller and simpler ones is called catabolism. And when it forms large molecules from smaller ones, it is called anabolism.
- Enzymes form an active site on which substrate comes and binds and this binding results in a much faster reaction. “Lock and key model” explains this activity.
- Enzymes lower the activation energy of the reaction, without altering the reactant or product proportions.
- Enzymatic mechanism;
𝑬 + 𝑺 ↔ 𝑬𝑺 ↔ 𝑬 + 𝑷- Substrate combines with the active site of the enzyme to form an enzyme- substrate complex.
- The activation energy is lowered and the substrate is altered in the form of product.
- The substrate (product) is released from the active site, enzyme can be reused.
Inhibition ̶ its types
The inhibition of an enzymatic activity occurs due to change in certain factors including pH, temperature and substrate concentration. The alteration in shape of an active site or the occupying of a foreign molecule on an active site may also cause inhibition.
- Competitive inhibition:
- When a molecule (competitive inhibitor), similar to the shape of substrate, tries to occupy the active site of the enzyme, it is called competitive inhibition.
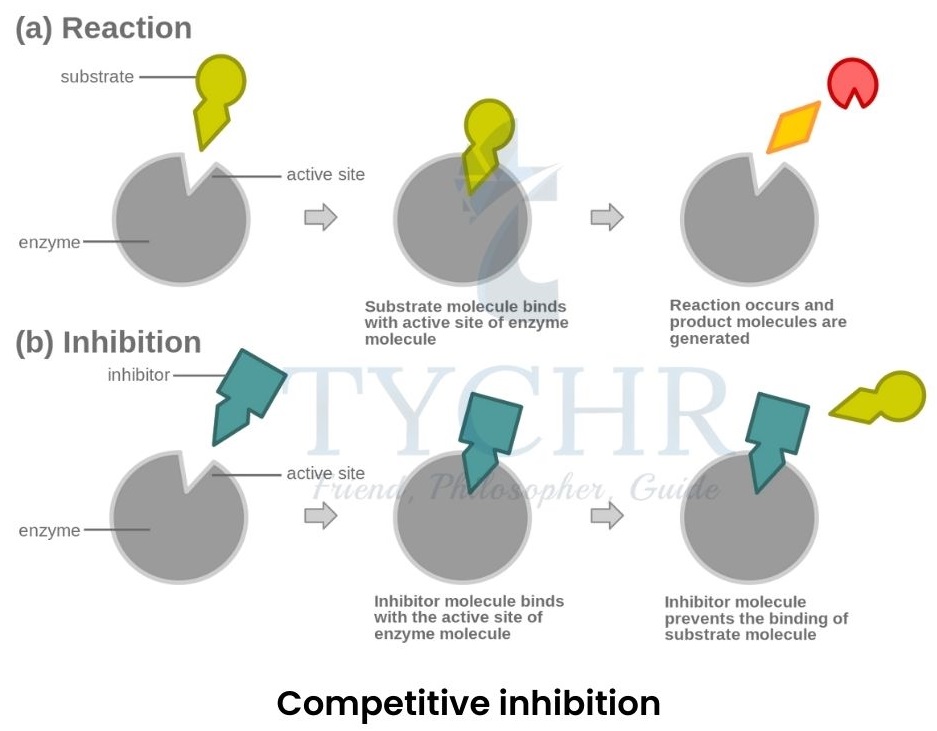
- Example: Taking sulfa drugs (sulphanilamide) can be helpful to kill bacteria during an infection. It tries to stop the production of paraminobenzoic acid (PABA) by blocking the enzyme which produces it. Stopped production of PABA further stops the formation of folic acid, which is a coenzyme of bacteria and causes death of the bacteria.
- When a molecule (competitive inhibitor), similar to the shape of substrate, tries to occupy the active site of the enzyme, it is called competitive inhibition.
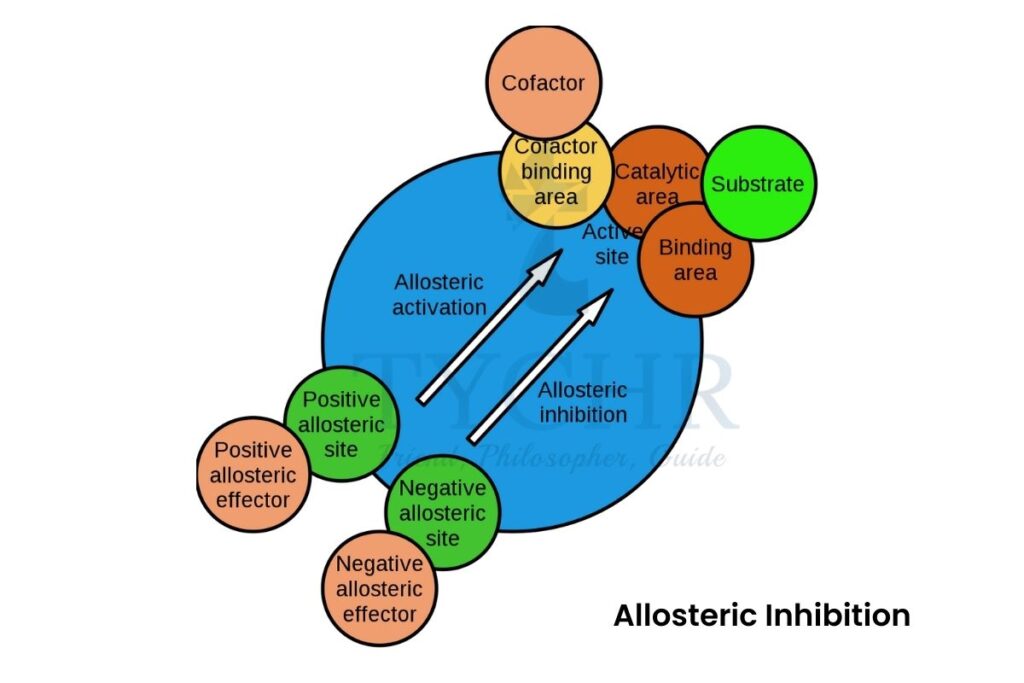
- Non-competitive inhibition:
- When a molecule (inhibitor) interacts with another site rather than the active site of the enzyme to change the conformation of its active site, then it is called non-competitive inhibition.
- The site it occupies is called an allosteric site and therefore it is also called allosteric inhibition.
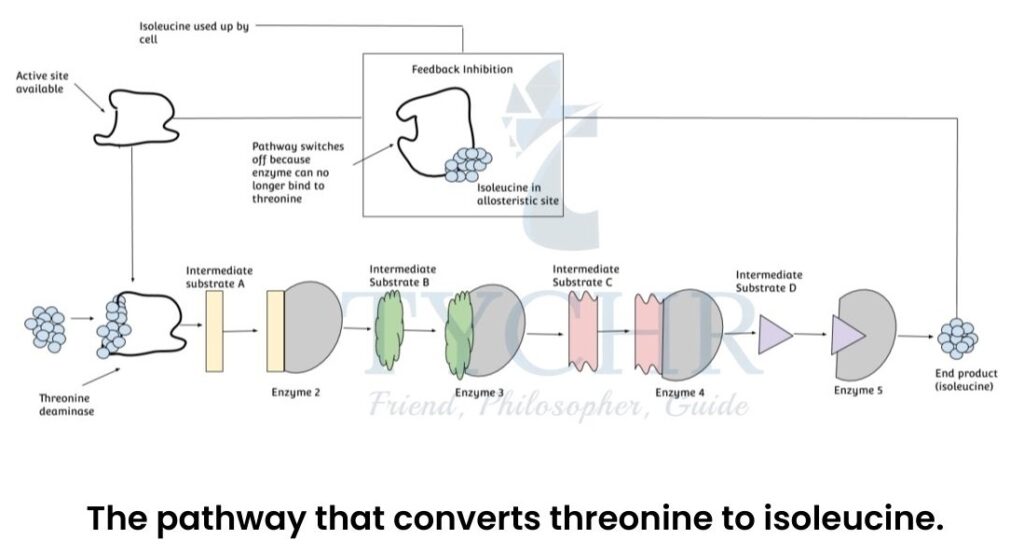
- End-product inhibition:
- Many metabolic reactions occur in an assembly-line type of process with each step catalysed by an enzyme to achieve the end product.
- When the product is present in sufficient amounts, the process stops.
- When the substance (actually a product) binds to the allosteric site of the enzyme in initial steps, it acts as inhibitor and thereby stops the further process, since the product is already achieved.
- Example: Pathway that converts threonine to isoleucine.
Cell respiration
Overview and process ̶ Substrate level phosphorylation
Cell respiration is a catabolic pathway.
- A general equation for this catabolic process is;
𝐶6 𝐻12𝑂6 + 6 𝑂2 → 6 𝐶𝑂2 + 6 𝐻2𝑂 + 𝑒𝑛𝑒𝑟𝑔𝑦
- This is a redox reaction. The oxygen atoms of the reactants get reduced due to the addition of two hydrogen atoms and the glucose is oxidised.
- The reduced molecule has much more potential energy than the oxidised form of molecule, therefore the energy is released when the glucose gets oxidised.
- Much of this released energy is evolved as heat and only 30% (of the energy in the chemical bonds of the glucose) is released as ATPs.
- Total 36 ATP molecules are produced by cellular respiration but the number comes to 30 ATPs because some of it gets utilised in the process itself.
- All the living organisms carry out respiration for energy production.
- Processes of respiration follows:
It follows the common pathway when initiated for all the organisms, referred to as
Glycolysis (‘lysis’ means to break).
Fate of glycolysis: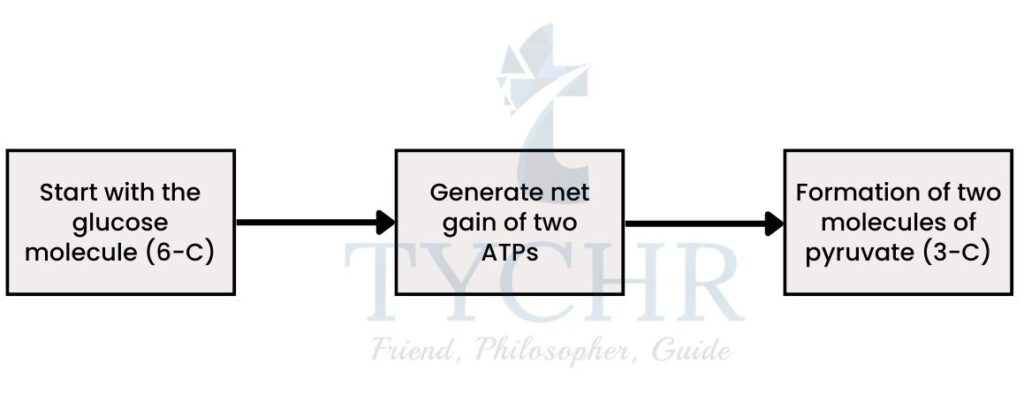
- Glycolysis is the method of slow oxidation of the glucose using various enzymes. It is a sequential process and at every step a bond is broken with the release of energy, which is released in the form of A
- If no oxygen is available, the pyruvate molecules enter the anaerobic pathway which is followed by fermentation. But if there is the presence of oxygen, then it follows aerobic pathway which further has three stages:
- link reaction
- Krebs cycle
- Oxidative phosphorylation
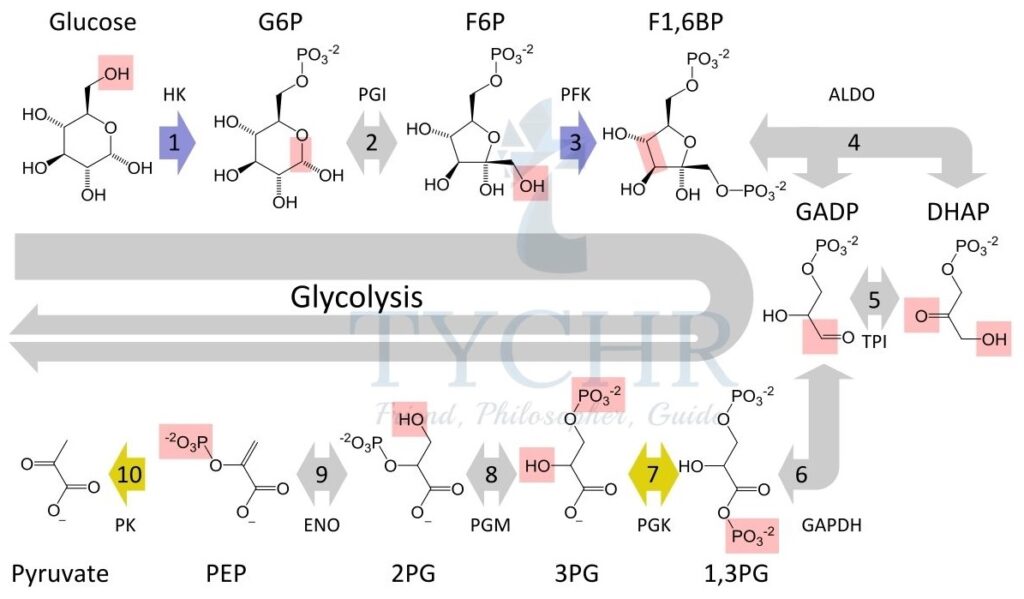
Glycolysis
- In this process of glucose breakdown, 4 molecules of ATP are generated and two are used in the process to conduct. So, net gain of 2 molecules of ATP occurs.
- 2 molecules of NADH are produced which is equivalent to 6 ATP molecules (1 NADH = 3 ATP).
- There is a direct transfer of a phosphate group from a phosphate-bearing molecule (phosphoenolpyruvate) to ADP in order to make ATP. The process is called substrate-level phosphorylation.
- It is an enzyme mediated process. Therefore its regulation is done by enzymes based on the amount of ATP molecules in the cytoplasm.
- The result would be two pyruvate molecules at the end of the cycle.
- This process occurs in both prokaryotic and eukaryotic cell and uses no oxygen i.e.
anaerobic.
Glycolysis
Link reaction and the Krebs cycle
- Pyruvate enters the matrix of the mitochondria and performs decarboxylation link reaction to form 2-C acetyl coenzyme A (acetyl CoA).
- Acetyl CoA usage depends upon the cellular ATP levels. If the ATP levels are low, then it will enter the Krebs cycle but if the ATP level is high enough, then it can be synthesised into lipid for storage purposes.
- Series of steps in Krebs cycle are:
- Acetyl CoA combines with oxaloacetate (4-C) and forms citrate (6-C).
- Citrate is oxidised to (5-C) by releasing carbon as CO2. NAD+ gets reduced to NADH.
- 5-C gets oxidised and decarboxylated to (4-C) and again reduces NAD+ to NADH.
- This 4-C compound undergoes many enzymatic steps to produce NADH, FADH2 and ATP molecules and then form oxaloacetate again, to start the cycle over again.
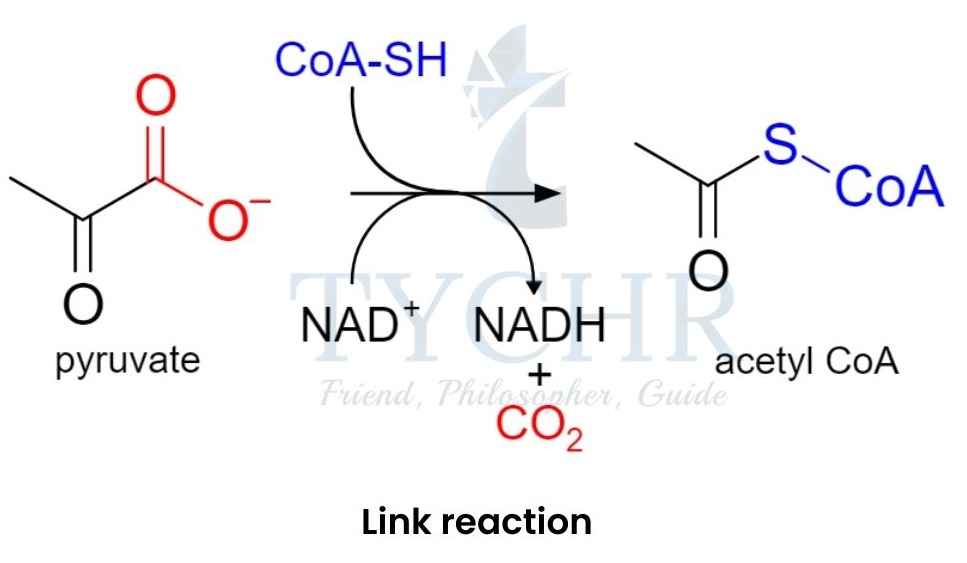
Figure 8.1 Link reaction 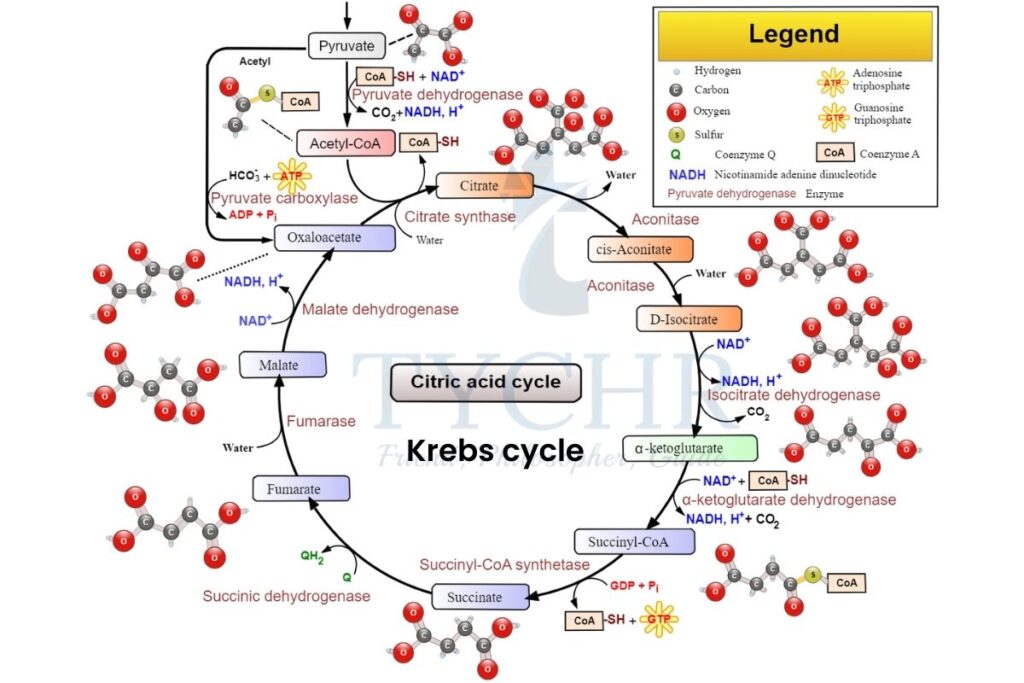
Figure 8.2 Krebs cycle
- Points to be noted:
- Krebs cycle will run twice for complete oxidation of one glucose molecule because one cycle processes one pyruvate molecule.
- From this cycle, 2 molecules of ATP, 6 molecules of NADH, 2 molecules of FADH2 are produced and 4 molecules (2 from link reaction + 2 from Krebs cycle) of CO2 are released.
- 4 molecules (2 from glycolysis) of ATP are produced until this cycle.
Electron transport chain (ETC) ̶ Oxidative phosphorylation
- It is the series of protein complexes called cytochromes (they contain haem group) and organic molecules to pass on the electrons from one member of the chain to another (due to energy gradient) in a series of redox reactions.
- These complexes are embedded in the inner membrane and in the cristae of the mitochondria.
- These embedded molecules are easily reduced or oxidised and therefore brings out redox reactions.
- The electron receiving molecule has higher electronegativity to attract the electrons, and when the electrons get transported, a certain amount of energy is released. This energy is harnessed by the cell to carry out phosphorylation.
- The coenzymes NADH and FADH2 released in previous cycles act as the sources of electrons.
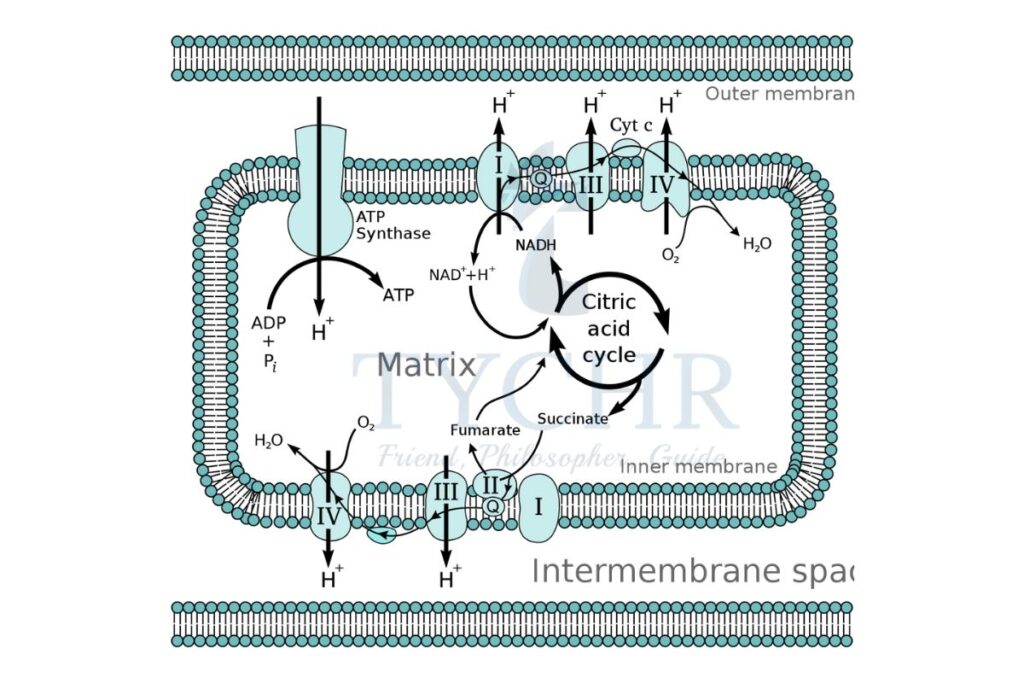
- NADH allows the production of three ATPs and FADH2 allows the production of 2 ATP molecules.
- At last, oxygen accepts electrons due to its high electronegativity which combines with the protons to form water.
- Energy difference between the embedded molecules allows the phosphorylation to occur in which the ADP molecule turns into ATP.
- Chemiosmosis is the process in which movement of protons occurs to provide energy for phosphorylation.
- An enzyme called ATP synthase is embedded in the inner membranes of the mitochondria which facilitates ions across.
- The energy for protons transfer from mitochondrial matrix to intermembrane space is provided by electrons.
- → The hydrogen ion gradient created due to this transfer causes the protons to transfer passively from intermembrane space to the matrix by ATP synthase.
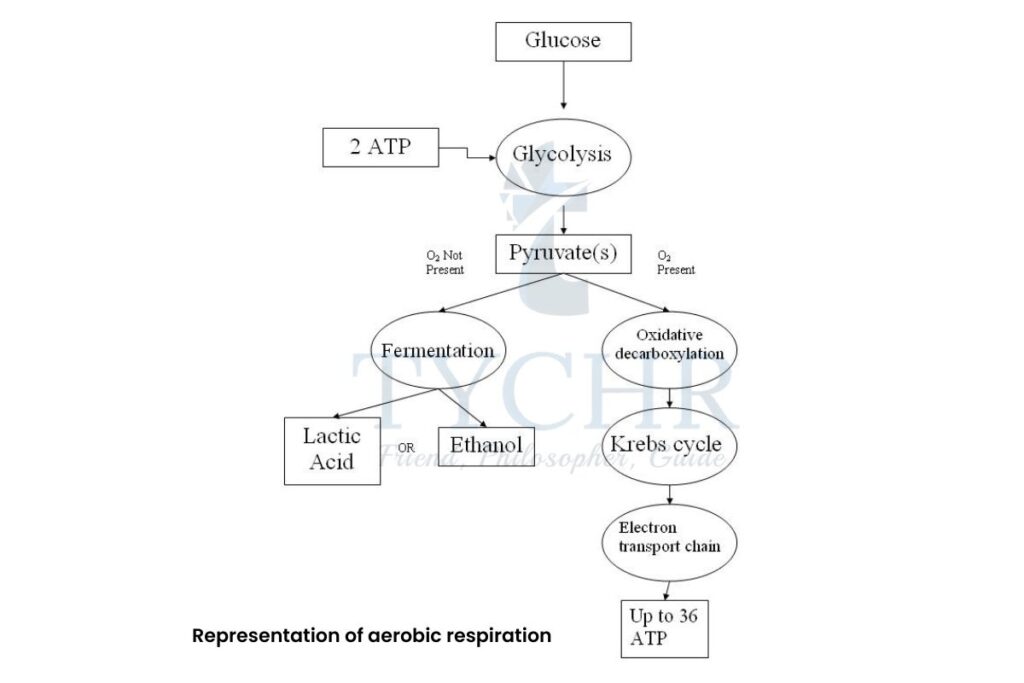
Figure 8.3 Representation of aerobic respiration
Photosynthesis
Overview and process
The conversion of the light energy into chemical energy is called photosynthesis. It forms the basis of food for every organism on the earth. Plants and certain bacteria are autotrophic which can use sunlight and inorganic matter to convert into useful organic matter (sugar). It is an anabolic process.
Equation: 6 𝐶𝑂2 + 12 𝐻2𝑂 → 𝐶6𝐻12𝑂6 + 6 𝐻2𝑂 + 6 𝑂2
How plants absorb the light energy?
- In the leaves of the plants, there is a presence of green colour organelle called chloroplast which contains the light absorbing pigment chlorophyll in it.
- Plants use the human visible spectrum of electromagnetic waves as a light to make food.
- Chloroplasts themselves are green in colour, therefore they reflect back green light and absorb red and blue light. This is the reason why plants show much more efficient photosynthesis in blue and red light.
- Photosynthesis completes in two stages:
- Light-dependent reactions
It is a non-cyclic reaction occurs in thylakoids and the stack of thylakoids is called granum.
→ Presence of pigments like chlorophyll, xanthophyll and carotenoids trap light energy and convert it into the chemical energy (ATP). - Organised structure of these pigments in thylakoid membrane is called photosystem. It includes chlorophyll a, accessory pigments and a protein matrix.
- Photosystem contains even more complex structures within the reaction centre. It contains chlorophyll molecules, protein matrix and a primary electron acceptor.
- Types of photosystem; Photosystem I and Photosystem II. Photosystem II (P680) works efficiently at 680 nm wavelength of light while the photosystem I (P700) works efficiently at 700 nm.
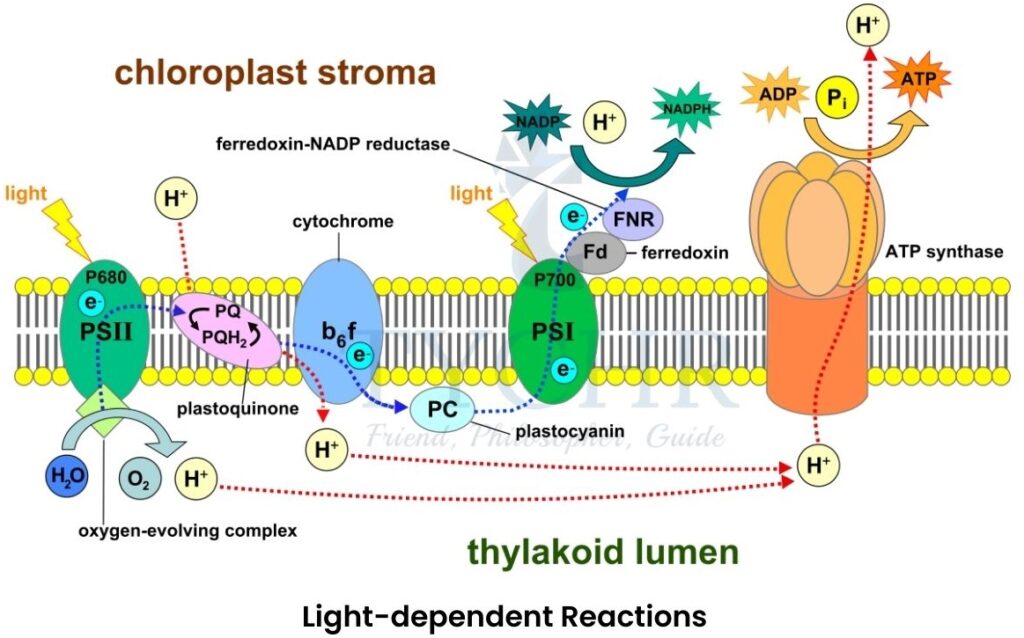
Figure 8.4 Light-dependent
- Light-dependent reactions
- Light-independent reactions
- It happens at night.
- It occurs in stroma of the chloroplast.
- ATP and hydrogen produced in the daylight is now used to convert CO2 and H2O into glucose.
6𝐶𝑂2 + 6𝐻2𝑂 → 𝐶6𝐻12𝑂6 + 6𝑂2
Glucose - It involves the Calvin cycle.
- The reactions occur as;
- RuBP combines with CO2 (carbon fixation catalysed by rubisco) and forms an unstable 6-C compound.
- Unstable 6-C compound is broken down into two glycerate 3-phosphate molecules which are then converted into triose phosphate (reduction).
- Now some of the triose phosphate molecules leave the cycle but some continue it to regain RuBP molecules (using ATP).
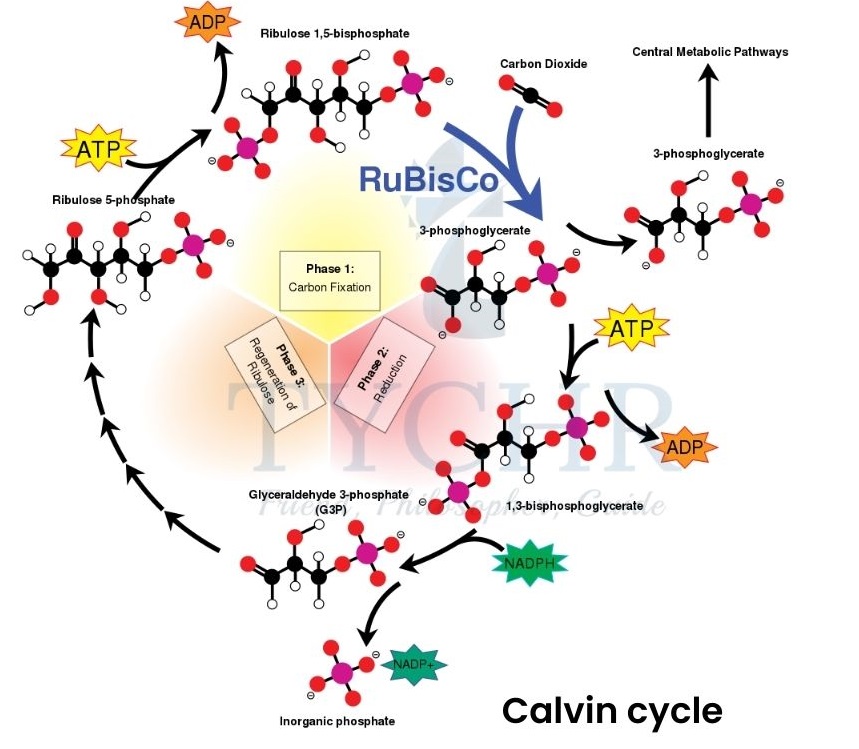
Figure 8.5 Calvin cycle
Points to be noted:
- One molecule of glucose and 6 molecules of RuBP is produced.
- 18 molecules of ATP and 12 molecules of NADPH are required to produce one molecule of glucose.
- Triose phosphate can be used to produce disaccharides and polysaccharides.

