IB Chemistry SL Paper 2 Question Bank
The IB Chemistry SL Paper 2 Question Bank is full of high-quality questions that will help you prepare for your IB Chemistry SL exam. The questions are divided into different topic areas, so you can focus on the areas that you need to work on the most. The question bank also includes answer explanations, so you can understand why the correct answer is correct. With the IB Chemistry SL Paper 2 Question Bank, you will have all the resources you need to ace your IB Chemistry exam!
Time: 1 hour 15 minutes
Instructions to candidates
- Answer all questions.
- Answers must be written within the answer boxes provided.
- A calculator is required for this paper.
- A clean copy of the chemistry data booklet is required for this paper.
- The maximum mark for this examination paper is [50 marks].
1.)
a.)
i.) Define the term electronegativity.
Electronegativity is defined as the ability of an atom to attract electrons towards itself in a covalent bond.
ii.) Arrange the following elements in order of increasing electronegativity: chlorine, nitrogen, fluorine.
The order of increasing electronegativity is: nitrogen < chlorine < fluorine.
b.) An experiment was conducted to determine the enthalpy change of a reaction. The following data was collected:
- Initial temperature: 25°C
- Final temperature: 30°C
Mass of solution: 100 g
Heat capacity of solution: 4.18 J/g·°C
Calculate the enthalpy change of the reaction.
The enthalpy change of the reaction can be calculated using the formula:
ΔH = q/m, where q is the heat absorbed by the solution, and m is the mass of the solution.
The heat absorbed by the solution can be calculated using the formula: q = mcΔT, where c is the heat capacity of the solution, and ΔT is the change in temperature.
Substituting the values given, we get:
q = (100 g) x (4.18 J/g·°C) x (30°C – 25°C) = 2090 J
ΔH = q/m = 2090 J/0.1 kg = 20,900 J/kg = 20.9 kJ/mol
2.)
a.)
i.) Define the term acid dissociation constant (Ka)
Acid dissociation constant (Ka) is a measure of the strength of an acid in solution. It is defined as the ratio of the concentrations of the products to the concentration of the acid, raised to the power of the number of acidic protons dissociated.
ii.) Write the expression for the Ka of acetic acid (CH3COOH)
The expression for the Ka of acetic acid (CH3COOH) is:
Ka = [H+][CH3COO-]/[CH3COOH]
iii.) Calculate the pH of a 0.1 M solution of acetic acid, given that Ka = 1.8 × 10-5.
To calculate the pH of a 0.1 M solution of acetic acid, we first need to calculate the concentration of H+ ions using the Ka value:
Ka = [H+][CH3COO–]/[CH3COOH]
1.8 x 10-5 = [H+][0.1]/[0.1]
[H+] = 1.34 x 10-3 M
Now, we can calculate the pH using the formula:
pH = -log[H+]
pH = -log(1.34 x 10-3)
pH = 2.87
Therefore, the pH of a 0.1 M solution of acetic acid is 2.87.
b.) The following reaction takes place in a closed system:
N2(g) + 3H2(g) ⇌ 2NH3(g)
i.) What effect does increasing the pressure have on the position of equilibrium? Explain your answer.
Increasing the pressure would shift the position of equilibrium towards the side with fewer gas molecules. In this case, the position of equilibrium would shift towards the side with fewer moles of gas, which is the right-hand side (the side with NH3). Therefore, increasing the pressure would increase the concentration of NH3.
ii.) What effect does increasing the concentration of nitrogen have on the position of equilibrium? Explain your answer.
Increasing the concentration of nitrogen would shift the position of equilibrium towards the side with fewer moles of gas, which is the left-hand side (the side with N2 and H2). Therefore, increasing the concentration of nitrogen would decrease the concentration of NH3.
3.) Urea is used as a fertilizer. It is secreted by mammals.
a.) The structural formula for urea is shown below. State the electron domain geometry and molecular geometry for the Nitrogen and Carbon atoms.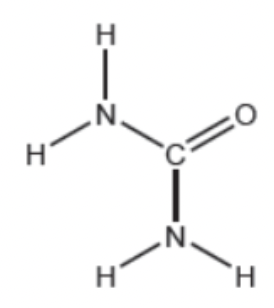
Answer:
Nitrogen has a tetrahedral electron geometry structure and a trigonal pyramidal molecular geometry structure. Whereas, carbon has a trigonal planar electron geometry structure and a trigonal planar molecular geometry structure.
b.) Urea can be made from a direct combination of ammonia and carbon dioxide gasses.
2NH3 (g) + CO2 (g) ⇌ (H2N)2CO (g) + H2O (g) Enthalpy < 0
Outline what happens to the equilibrium constant when the temperature is increased
Answer:
Since the reaction is exothermic (enthalpy is lesser than 0), the reverse reaction will be favored and the equilibrium constant will decrease.
c.) Urea combustion produces water, carbon dioxide and nitrogen. Write a balanced equation for the reaction with the state symbols.
Answer:
2(H2N)2CO (s) + 3O2 (g) → 4H2O (l) + 2CO2 (g) + 2N2 (g)
Balanced equation means the total number of moles are equal on both sides. The number of moles on the LHS add up to 12 → [(2 x 2) + 2 + (3 x 2)] = 12.) For the RHS, it also adds up to 12 → [4 + (2 x 2) + (2 x 2)]
4.) This question is regarding periodicity.
a.) Define first ionization energy
Answer:
The first ionization energy is the energy required to remove one mole of the most loosely held electrons from one mole of gaseous atoms to produce 1 mole of gaseous ions each with a charge of 1+
b.) Explain why the first ionization energy of silicon is higher than aluminum
Answer:
It is harder to remove the outer electron from Silicon because silicon has 1 more proton than aluminum so has stronger proton charge. However, the electrons are in 3p level so have the same amount of electron shielding. Therefore, the electrostatic force between the nucleus and the valence electrons is higher in silicon than in aluminum.
c.) Explain why sodium oxide has a higher melting point than sulfur trioxide
Answer:
Na2O is ionic therefore it has a stronger electrostatic attraction between Na+ and O2-. SO3, on the contrary, has weak intermolecular forces/London dispersion forces which tend to be easily broken and are weaker than ionic bonds.
d.) Sodium oxide and sulfur trioxide are added to separate beakers containing water. State the equation for each of the reactions and identify the acidity of each of the oxides.
Answer:
Sodium oxide: Na2O (s) + H2O (l) → 2NaOH (aq) – Sodium hydroxide, so basic
Sulfur trioxide: SO3 (l) + H2O (l) → H2SO4 (aq) – Sulfuric acid, so acidic
5.) 0.338 grams of methanol was combusted in a spirit burner. The heat released in the reaction increased the temperature of the 50 cm3 of water by 23.9 degree Celsius.
a.) Calculate the enthalpy change of combustion of the methanol
Answer:
First, the energy of the water needs to be calculated: q = mcΔT
Q = 0.0500 x 4.18 (specific heat capacity of water) x 23.9 = 4.9951 kJ
Finding the number of moles of methanol: 0.338/32.05 = 0.010546 = 1.05 x 10-2 mol
Therefore, enthalpy change = 4.9951/1.05 x 10-2 = 473.648 kJ/mol = – 4.73 x 102
It is a minus sign because it is an exothermic reaction (the temperature is released)
b.) Methanol can be manufactured using the following equation reaction:
CO (g) + 2H2 (g) CH3OH (g) Enthalpy < 0
State and explain the effect of the following changes on the equilibrium position of the reaction above
i.) Increase in temperature
Answer
Since the enthalpy is lesser than 0, it indicates an exothermic reaction. Therefore, the equilibrium will shift to the reactants side or it will favor the backwards reaction
ii.) Increase in pressure
Answer
Increasing the pressure will shift the equilibrium to the side with fewer gas moles, therefore the equilibrium will shift to the right as it has only 1 mole compared to the products side with 3 total moles.
iii.) Catalyst addition
Answer
Adding a catalyst has no effect on the equilibrium of the reaction. The rate of the backward and forward reaction will increase equally.
https://www.iitianacademy.com/ib-dp-chemistry-slhl-question-bank/


