IB Chemistry HL Paper 2 Question Bank
The IB Chemistry HL Paper 2 Question Bank is an essential tool for students preparing for the IB Chemistry HL exam. The question bank provides a wide range of practice questions, covering all aspects of the IB Chemistry syllabus. The questions are designed to challenge and test students’ understanding of the concepts they have learned. The question bank is an invaluable resource for students, helping them to prepare fully for the rigours of the IB Chemistry HL Paper 2 exam.
Time: 2 hours and 15 minutes
Instructions to candidates
- Answer all questions.
- Answers must be written within the answer boxes provided.
- A calculator is required for this paper.
- A clean copy of the chemistry data booklet is required for this paper.
- The maximum mark for this examination paper is [90 marks].
Answer all questions. Answers must be written within the answer spaces provided.
1.) The most common hydride of phosphorus is called Phosphine. It has a formula PH3
a.) Draw a Lewis structure of Phosphine
Answer: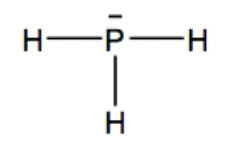
Since Phosphine is PH3, one phosphorus atom is in the middle connected by 3 hydrogen atoms around it. The line above the phosphorus atom shows the lone pair of electrons that has not been bonded with any other atom.
b.) State the hybridization of the phosphorus atom in Phosphine and its molecular structure
Answer:
The hybridisation of the phosphorus atom in phosphine is sp3, which means it has three bonds with hydrogen atoms. PH3 also has a trigonal pyramidal structure. The trigonal pyramid geometry is formed when the central atom is attached to three atoms and contains one lone pair.
c.) Determine if whether Phosphine would act as a Lewis base or a Lewis acid or neither
Answer:
Phosphine would act as a Lewis base because it is capable of donating a pair of electrons. A Lewis base is an electron-pair donor. PH3 has a pair of nonbonding electrons and can act as a donor.
d.) Define a Bronsted Lowry base
Answer:
A Bronsted-Lowry base is a substance which accepts a proton or H+ ion from the other compound and forms conjugated acid.
2.) The properties of the elements can be deduced from where they are placed on the periodic table.
a.) Explain why the atomic radius reduces as you go across Period 3.
Answer:
The nuclear charge or the number of protons increase as you go across Period 3. Therefore, when the nucleon number increases, it causes a stronger pull on the outer electrons. The electrostatic force (force acting between the electrons and the nucleus) increases therefore causing the size (atomic radius) of the atom to reduce.
b.) The periodic table also shows the relationship between the properties of elements and their electronic configuration
i.) Define electronegativity
Answer:
The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity.
ii.) State two reasons why metal ions have a smaller radius than metal atoms
Answer:
The ions tend to have a greater net positive charge. They would be pulling fewer electrons with the same number of protons therefore causing the ionic radius to decrease further.
iii.) The ionic radius of P3- is larger than that of the ionic radius of Si4+ Outline why.
Answer:
P3- has one more energy level than Si4+ so its valence electrons will be further from the nucleus and it will have a larger ionic radius.
3.) Consider the following reaction that is in equilibrium
2SO2 (g) + O2 (g) ⇌ 2SO3 (g) Enthalpy = -198 kJ/mol
a.) Explain how the conditions can be altered to increase the yield of the products
Answer:
Since it is an exothermic reaction, the temperature of the reaction can be reduced.) Additionally, the pressure on the yield of the sulfur trioxide can be increased (when pressure increases on the product side of the reaction with fewer moles, the yield also increases.
b.) State how the catalyst would affect the forward and reverse reaction, position of the equilibrium and the value of the equilibrium constant
Answer:
A catalyst would increase the rate of reaction of both the forward and backward reaction equally. Since it increases equally, and because catalysts don’t affect the yield produced in the reaction, there is no effect on the position of the equilibrium. Consequently, it does not affect the equilibrium constant either.
c.) Electroplating is one of the important applications of electrolysis.
i.) State the composition of the electrode and the electrolytes used in silver electroplating process
Answer:
Cathodes i.e. the negative electrodes, are the objects that are plated in the silver electroplating process. Objects that are made of steel such as a spoon. As for the anodes i.e. the positive electrodes, it is silver. This silver will be coated on the steel spoon. The electrolyte is Sodium argentocyanide solution or silver nitrate.
ii.) Outline two differences between a voltaic cell and an electrolytic cell
Answer:
A voltaic cell converts chemical energy to electrical energy whereas an electrolytic cell converts electrical energy to chemical energy. Furthermore, in an electrolytic cell, the cathode is the negative electrode and the anode is the positive electrode. However, it is the opposite for a voltaic cell – the anode is the negative and the cathode is positive.
4.)
a)
i.) Define the term “ligand” and explain how the strength of the ligand affects the stability of transition metal complexes.
Answer:
A ligand is a molecule or ion that can donate a pair of electrons to a transition metal ion to form a coordinate covalent bond.) The strength of the ligand affects the stability of transition metal complexes because the stronger the ligand, the greater the energy required to break the bond between the ligand and the metal ion, making the complex more stable.
ii.) Explain the difference between a coordination complex and a complex ion.
Answer:
A coordination complex consists of a central metal ion or atom surrounded by ligands that are bound to it through coordinate covalent bonds. A complex ion, on the other hand, is an ion that consists of a metal ion or atom bonded to one or more ligands.
iii.) Describe the factors that affect the color of transition metal complexes.
Answer:
The color of transition metal complexes is affected by several factors, including the nature of the metal ion, the oxidation state of the metal ion, the type and strength of the ligands, and the geometry of the complex. For example, transition metal complexes with d-electron configurations that are partially filled can absorb visible light and appear colored.
iv.) Discuss the importance of redox reactions in the chemistry of transition metals.
Answer:
Redox reactions play an important role in the chemistry of transition metals. Transition metals can undergo oxidation and reduction reactions, allowing them to form complexes with a range of oxidation states. These reactions are important in catalysis, biological processes, and industrial applications.
b)
i.) Explain the concept of chelation and give an example of a chelating agent.
Answer:
Chelation is the process by which a ligand forms a complex with a metal ion by binding to it through multiple coordination sites. EDTA (ethylenediaminetetraacetic acid) is an example of a chelating agent.
ii.) Discuss the properties of the transition metals that make them useful as homogeneous catalysts.
Answer:
Transition metals are useful as homogeneous catalysts because they have the ability to form multiple oxidation states and can coordinate with different ligands, allowing them to catalyze a wide range of reactions. Additionally, they often have high catalytic activity and selectivity.
iii.) Describe the role of transition metals in biological systems, giving examples of their functions in enzymes and proteins.
Answer:
Transition metals play important roles in biological systems, often as cofactors in enzymes and proteins. For example, iron is a component of heme, which is found in hemoglobin and myoglobin and is involved in oxygen transport in the blood.) Copper is a cofactor in the enzyme cytochrome c oxidase, which is involved in cellular respiration.
5.)
a.)
i.) Define the term “rate constant” in terms of a chemical reaction mechanism.
Answer:
The rate constant is the proportionality constant in the rate equation that relates the concentration of reactants to the rate of the reaction. It is specific to a particular reaction mechanism and reflects the probability of reactant collisions leading to a chemical reaction.
ii.) Explain the effect of temperature on the rate constant of a reaction.
Answer:
The rate constant increases with increasing temperature, as more molecules have sufficient energy to overcome the activation energy barrier and react. This is described by the Arrhenius equation.
iii.) How does the activation energy influence the rate constant of a reaction?
Answer:
The activation energy influences the rate constant by determining the fraction of collisions with sufficient energy to react. A higher activation energy results in fewer collisions with sufficient energy, leading to a lower rate constant.
iv.) This reaction follows a two-step mechanism:
First step: 2NO (g) ⇌ N2O2 (g) – fast
Second step: N2O2 (g) + O2 (g) ⇌ 2NO2 (g) – slow
Deduce the rate expression for the mechanism
Answer
The Rate constant for the first step is Kc = [N2O2] / [NO]2
[N2O2] = Kc [NO]2
From the second step, which is the slow step, rate = k1[N2O2][O2]
Replacing [N2O2] with [NO]2 with reference to the fast reaction
Rate = k2 K[NO]2[O2] = k[NO]2[O2]
b.)
i.) The rate constant of a second-order reaction is 1.5 x 10-3 L/mol.s at 25°c. If the activation energy for the reaction is 80 kJ/mol, calculate the rate constant at 40°C.
Answer:
Using the Arrhenius equation, we can calculate the rate constant at 40°C:
k2 = k1 * exp[(Ea/R) * ((1/T2) – (1/T1))]
k2 = 1.5 x 10-3 * exp[(80,000 J/mol) / (8.314 J/mol.K) * ((1/313 K) – (1/298 K))]
k2 = 4.01 x 10-3 L/mol.s
ii.) The hydrolysis of ethyl acetate (CH3COOC2H5) is a first-order reaction with a rate constant of 2.5 x 10-4s-1 at 25°c. Calculate the time required for the hydrolysis of 0.25 moles of ethyl acetate in a 2 L solution at 25°C.
Answer
The rate of the reaction is given by the first-order rate equation: rate = k [ethyl acetate].
Rearranging, we get: [ethyl acetate] = initial [ethyl acetate] * exp(-kt). Substituting the values, we get: 0.25 mol / 2 L = initial [ethyl acetate] * exp(-2.5 x 10-4s-1*t)
Solving for t, we get:
t = ln(0.25 mol / 2 L) / (-2.5 x 10-4s-1)
t = 3,468 seconds or 57.8 minutes
6.) This question is about carbon and chlorine compounds
a.) A reaction happens between Ethane (C2H6) and the chlorine in the sunlight. State and briefly explain what this reaction is and the name of the mechanism that occurs during the reaction
Answer:
It is a substitution reaction and the mechanism is free-radical.
Ethane reacts with chlorine by free radical halogenation in the presence of sunlight when chlorine breaks down to form two chlorine radicals. The chlorine radical reacts with ethane to give ethane radical which reacts with other chlorine to generate a halogenated product C2Cl6.
b.) A possible product that results from the reaction between ethane and chlorine has the following composition in terms of percentage mass:
Carbon: 24.27% Hydrogen: 4.08% Chlorine: 71.65%
Determine the empirical formula
Answer:
Carbon: Atomic mass = 12.01 C = 24.27 / 12.01 = 2.021 2 moles
Hydrogen: Atomic mass = 1.01 C = 4.08 / 1.01 = 4.04 4 moles
Chlorine: Atomic mass = 35.45 C = 71.65 / 35.45 = 2.021 2 moles
Hence, C2H4Cl2 is the molecular formula
Empirical formula is the simplified molecular formula = CH2Cl


