As Seen On





















Google reviews
Hear from our International Students and Alumni…
Our Achievements
Why Choose TYCHR over the rest?
Choose TYCHR for unparalleled expertise and personalized support, setting us apart as your premier educational partner.
| Tychr | Other online tutoring agencies | Tuition centers (group sessions) | Private tutors | |
| IB expertise | Trained IB Teachers and Examiners up-to-date on IB syllabus changes and requirements. | Limited IB specialisation. May do IB as a side-stream or part time. | Limited IB specialisation. May do IB as a side-stream or part time. | Limited IB specialisation. Ex Students with limited resources and professional experience |
| Tutor quality | A coterie of passionate and dedicated tutors that can help you ace that 7. | Tutors from different backgrounds without proper knowledge of IB | Tutors from different backgrounds without proper knowledge of IB | Some really great tutors! No screening processes or compliance to guidelines |
| Access to your tutor | One-on-one access during group o Individual sessions. Easy access outside of sessions through mail or meetings too! | May be difficult to contact tutors on urgent basis or to schedule sessions | Limited access to your tutor in a group setting. | One-on-one access during sessions to focus on what you want. Easy access outside of sessions too! |
| Free Trials | We provide free trial sessions for the student to assess the class quality | May or may not be available | May or may not be available | May or may not be available |
| Value for money | Excellent quality sessions for a moderate price. | Moderate-to-high quality sessions for a high price. | High quality, but highly inflexible and impersonal sessions, for a reasonable price. | Variable quality sessions for variable prices. |
How It Works?
Follow the well-grounded 6 step process to uncontested success


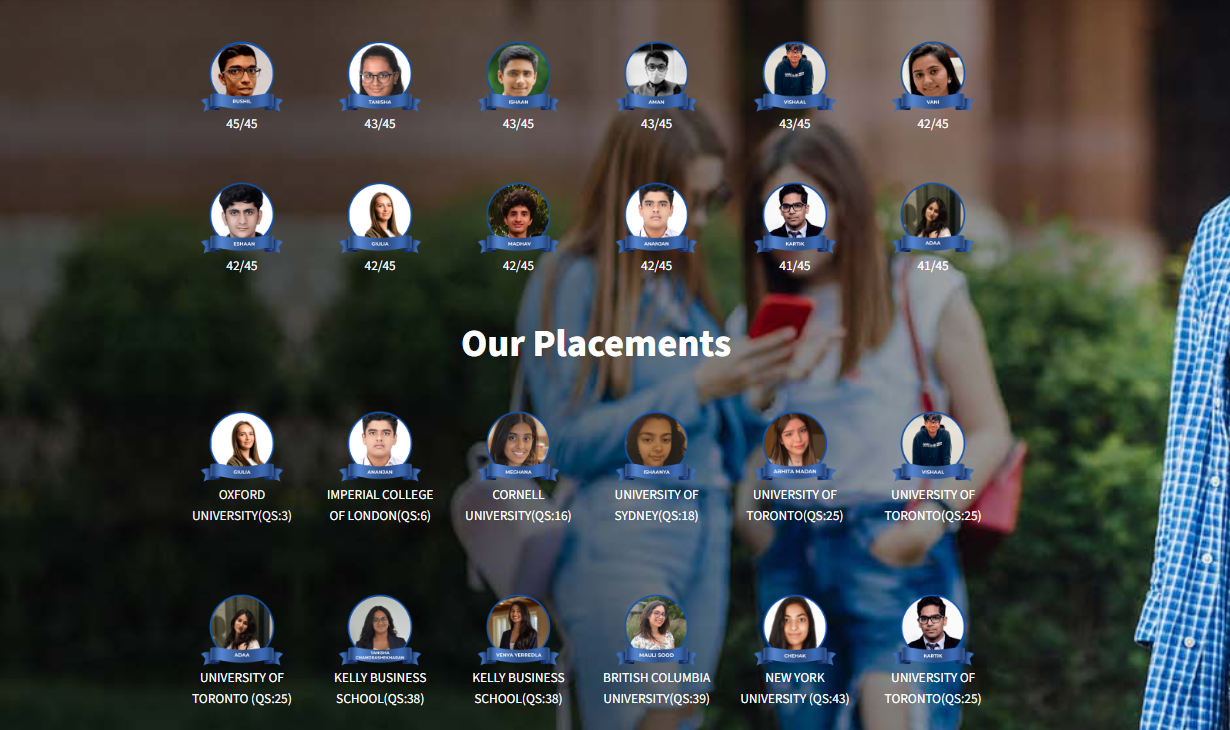
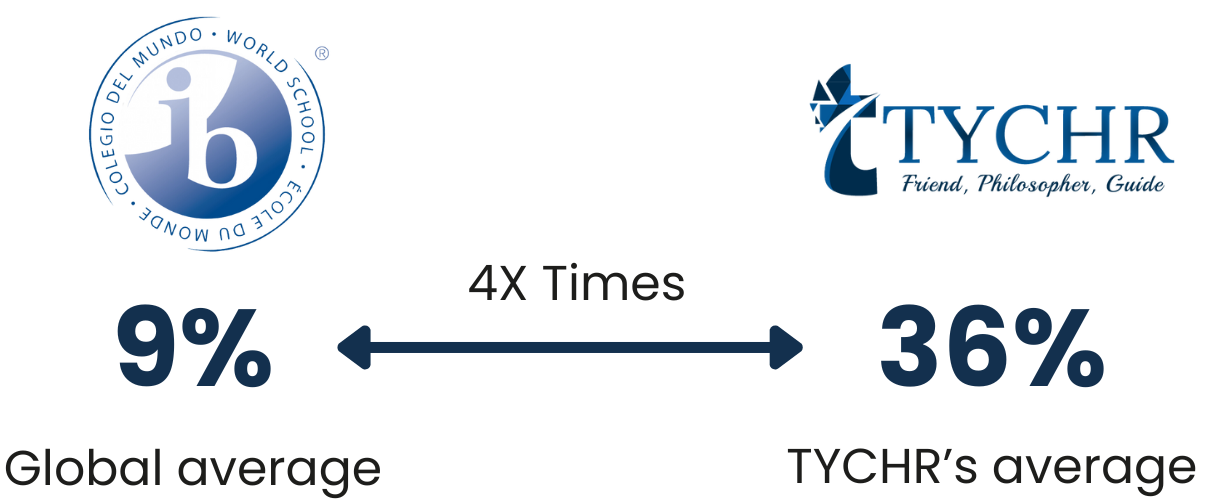
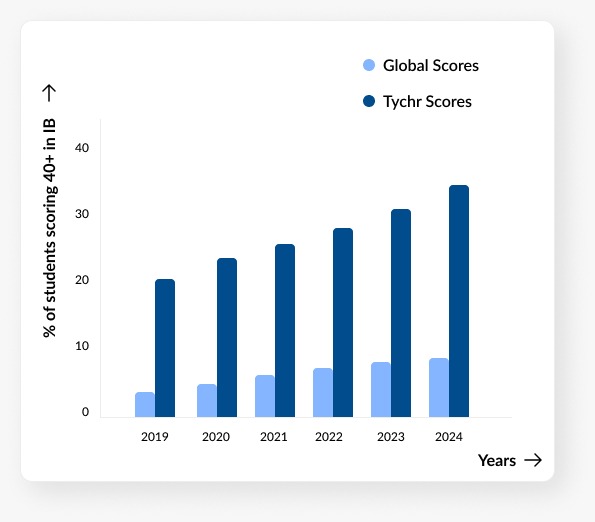
OUR 2024
Results
Measuring our results:Making sure we stamp out self doubt and pave the way
book your Free session now!
Test Methodlogy
Ready To Reach New Heights? Let’s Get There, Together.

Theoretical Framework
In the IBDP, experienced educators deliver personalized instruction to small groups of up to five students, offering high-quality resources across diverse subjects for a rigorous academic experience.
Explanatory Videos
After learning the basics, students are shown a specific collection of instructional videos. These videos are meant to help them master the foundational concepts and understand the curriculum better.
Strengthening Concepts
Students deepen their understanding through targeted assignments and personalized mentorship. Tychr also organizes global brainstorming sessions to support Extended Essay and TOK projects.
Time-Bound Test Series
Students take timed assessments simulating real exams, receiving graded scripts and detailed keys. Tychr’s analytical feedback offers performance insights, comparing their results with those of international peers.
STUDENTS ASSOCIATED
SCHOOLS COVERED
COUNTRIES SERVED
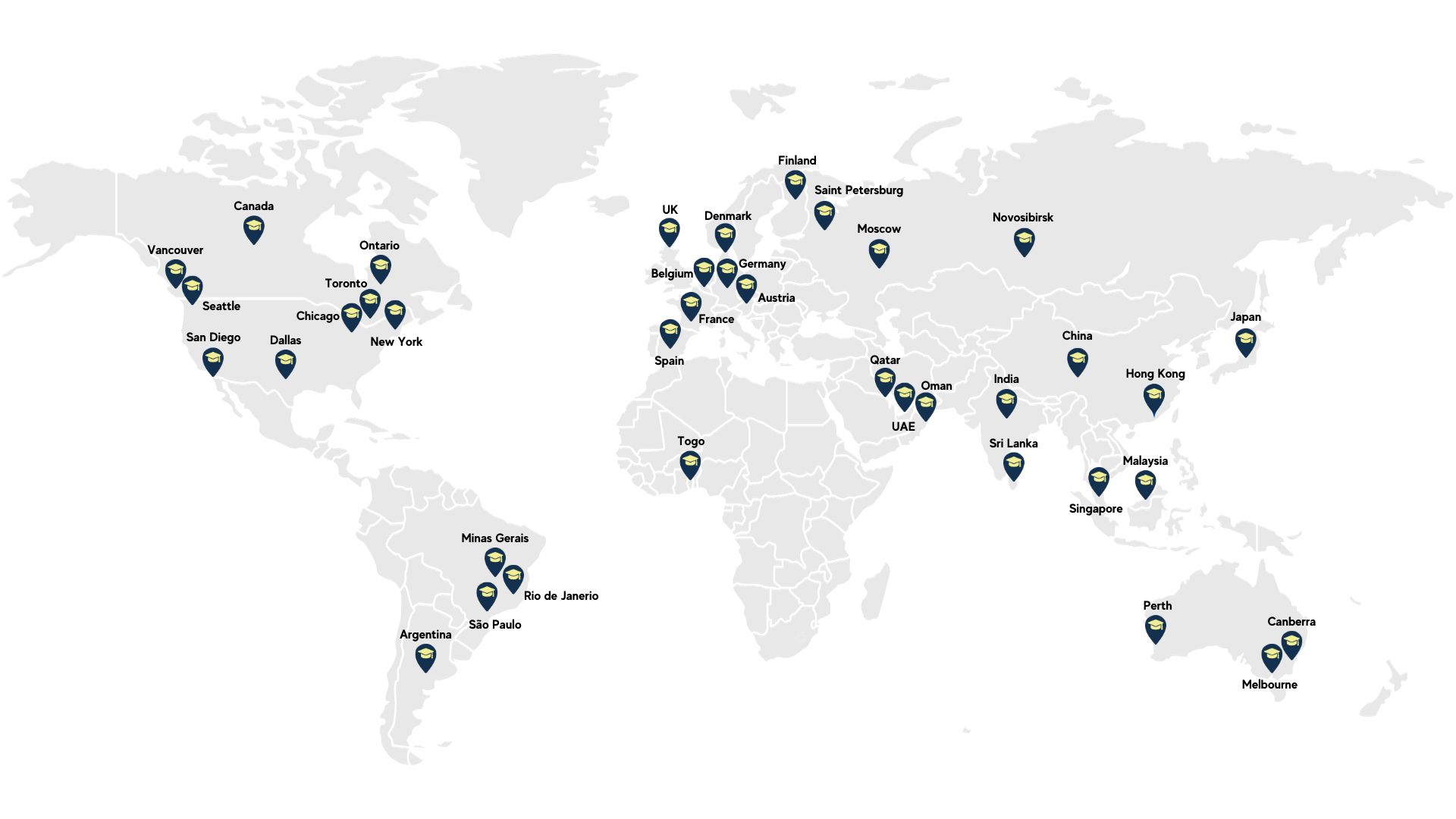
TYCHR Across the World
Trusted by International Clients in over 70+ countries with unparalleled tutoring experience
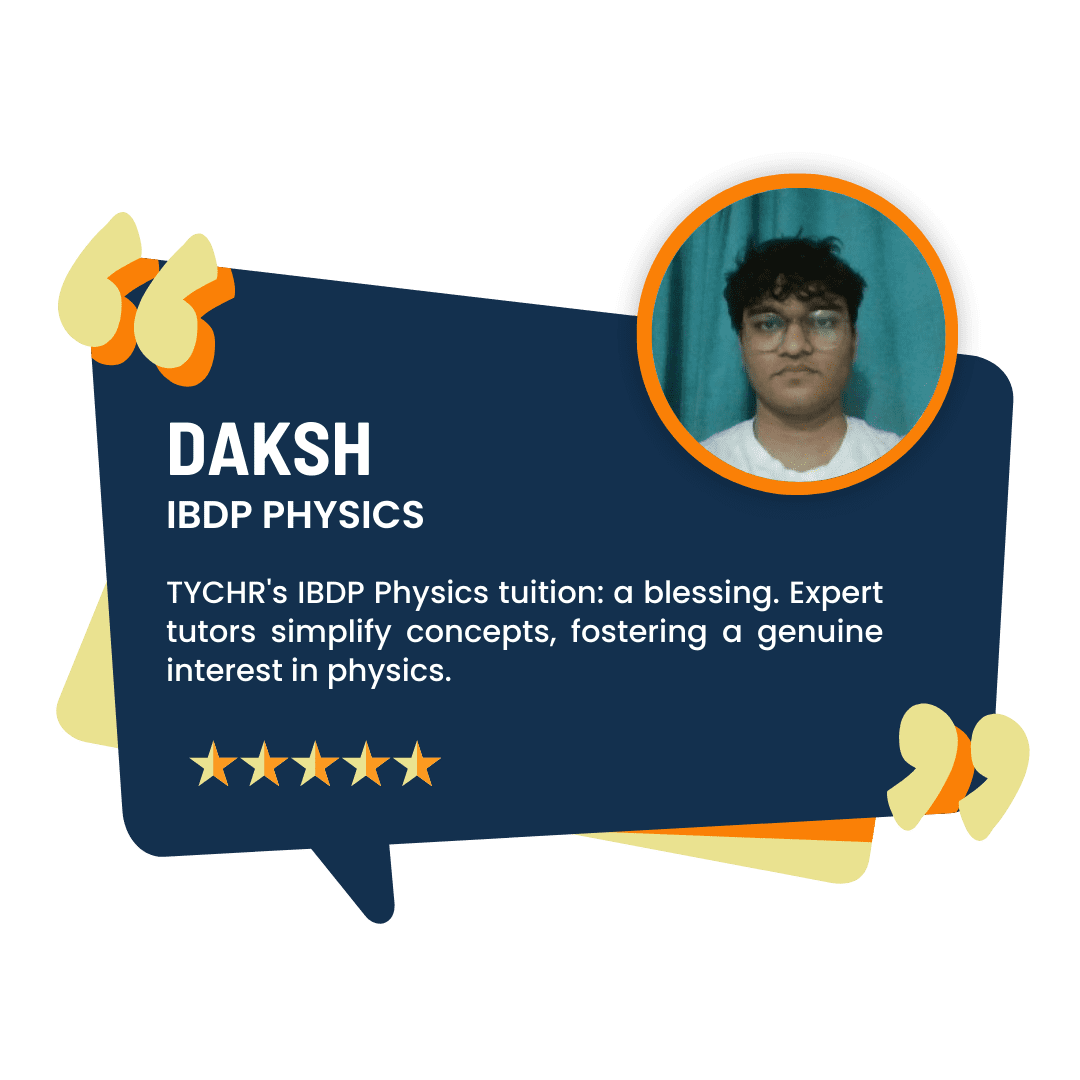
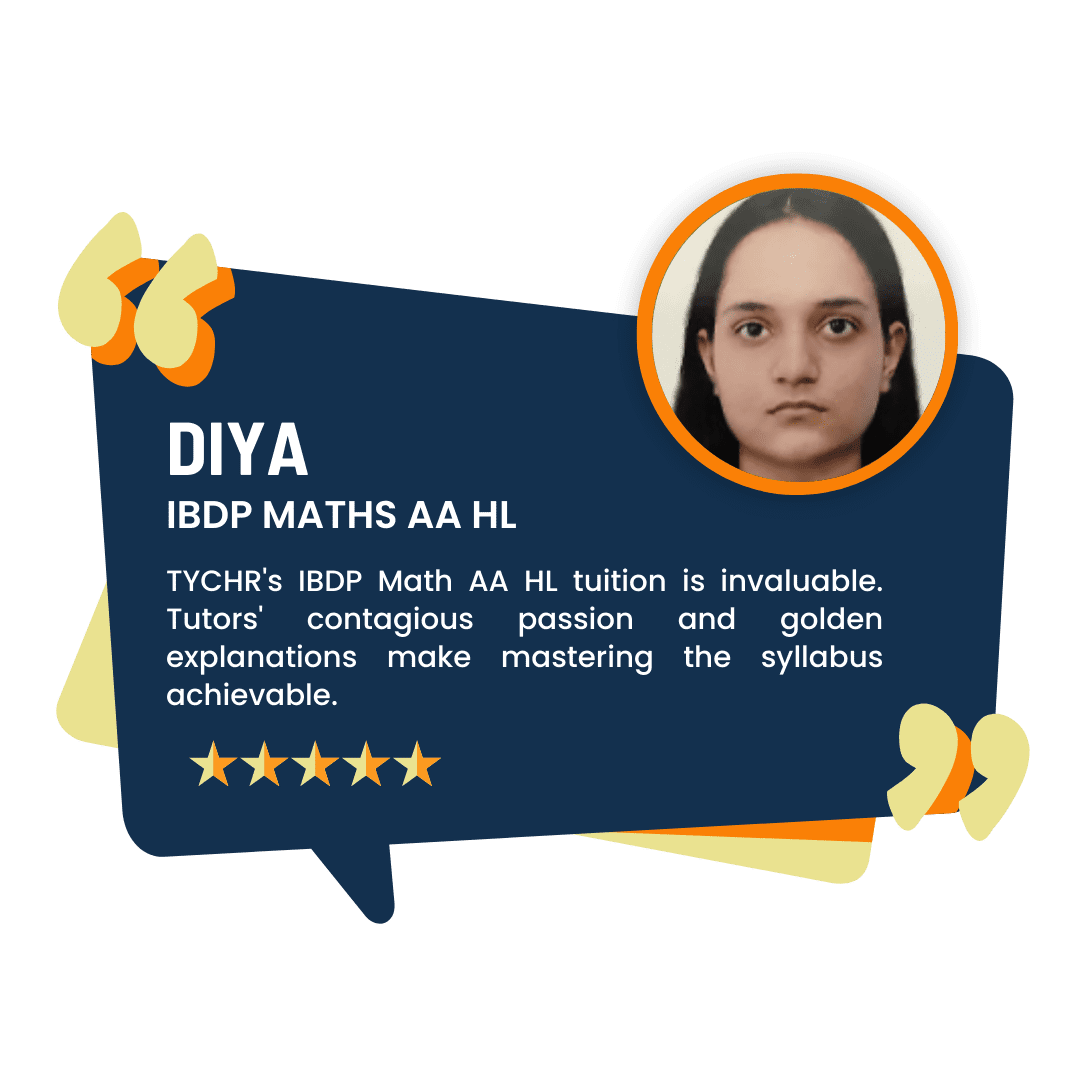
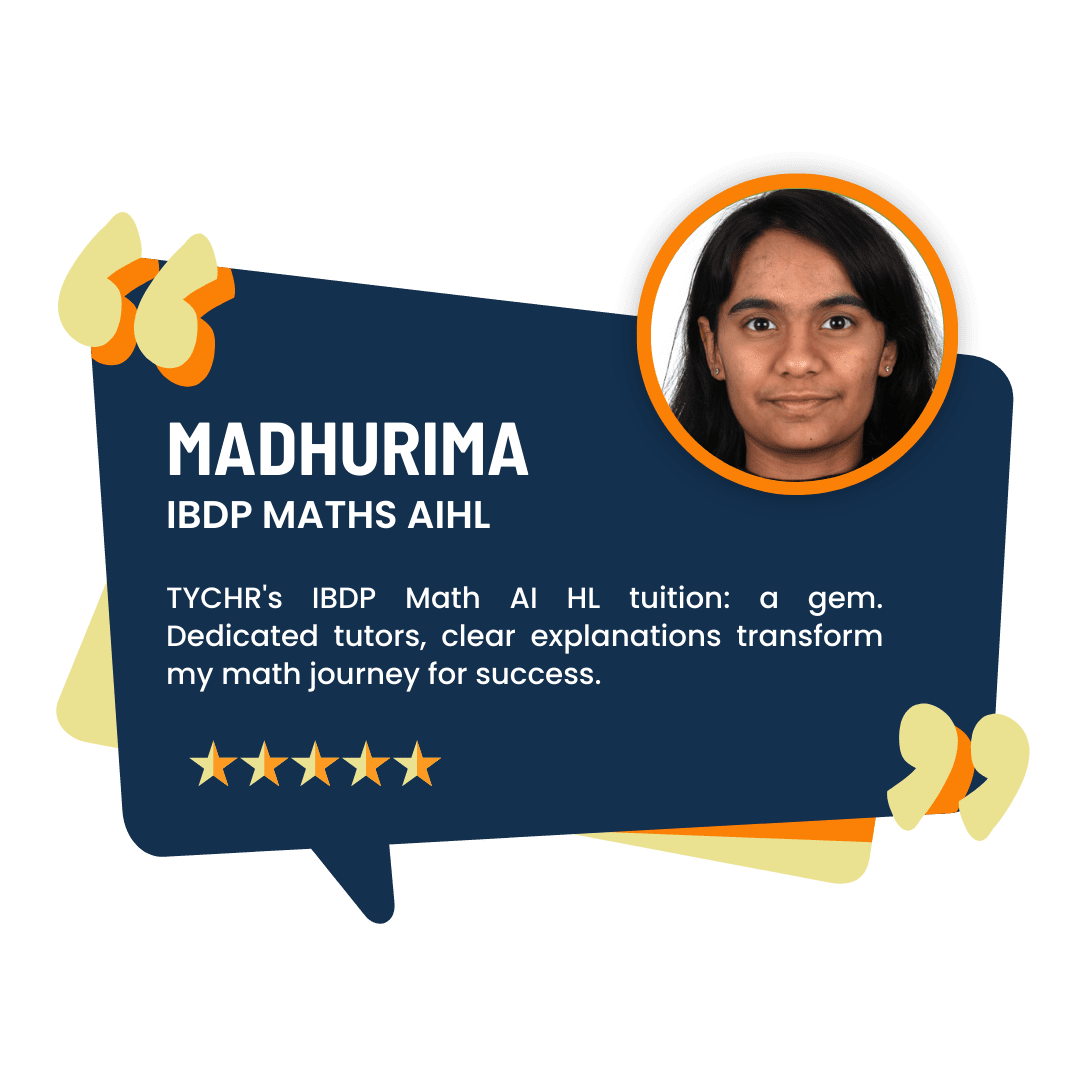
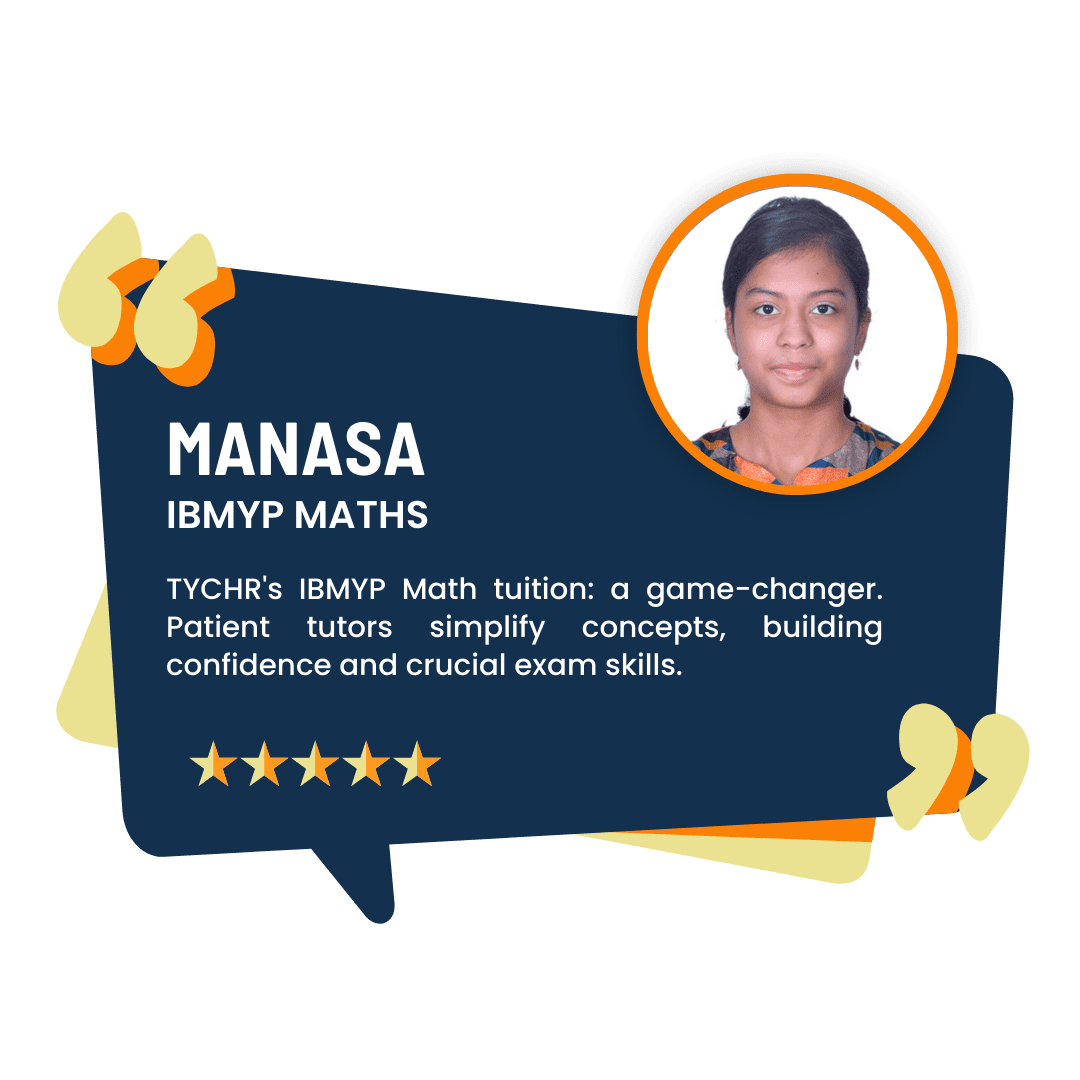
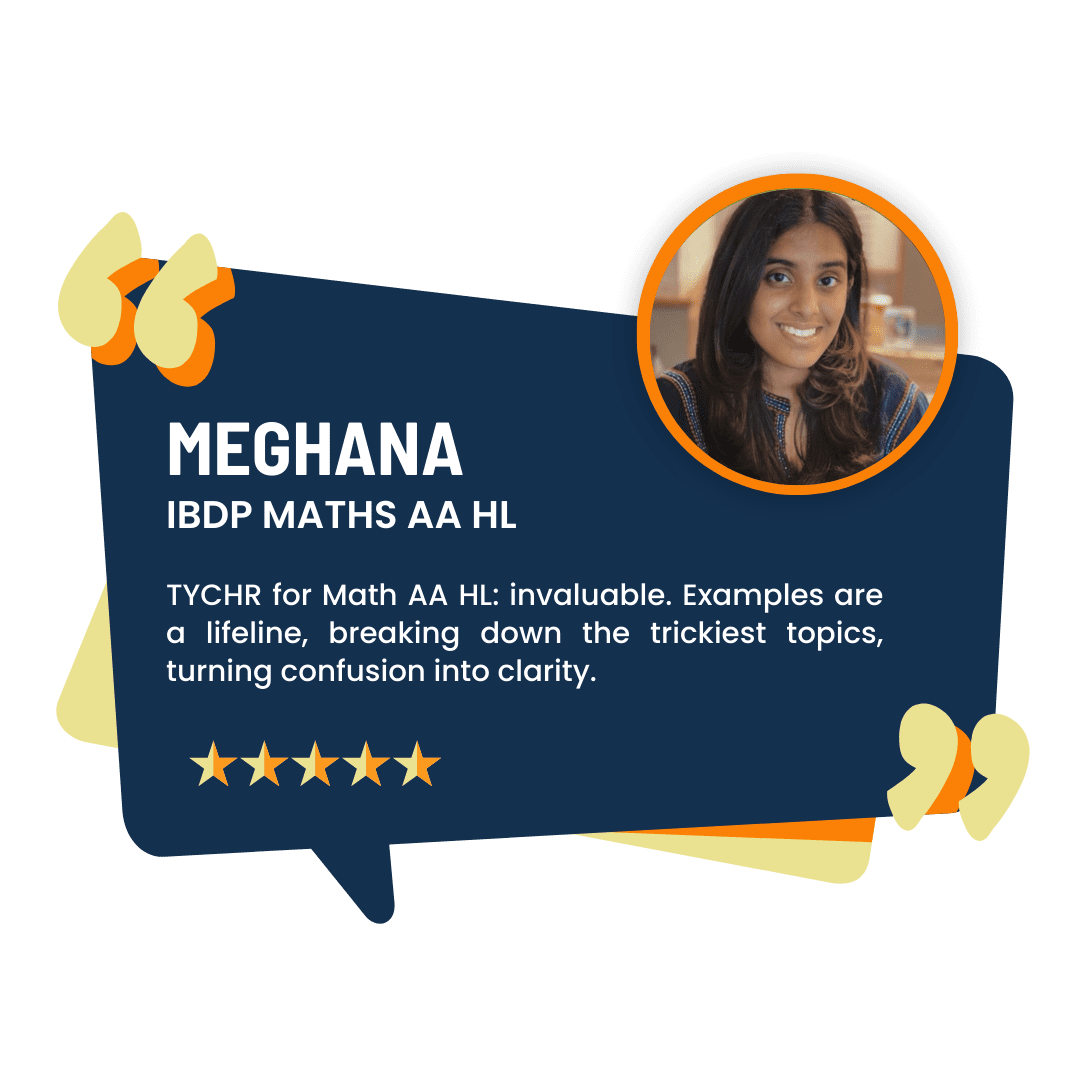
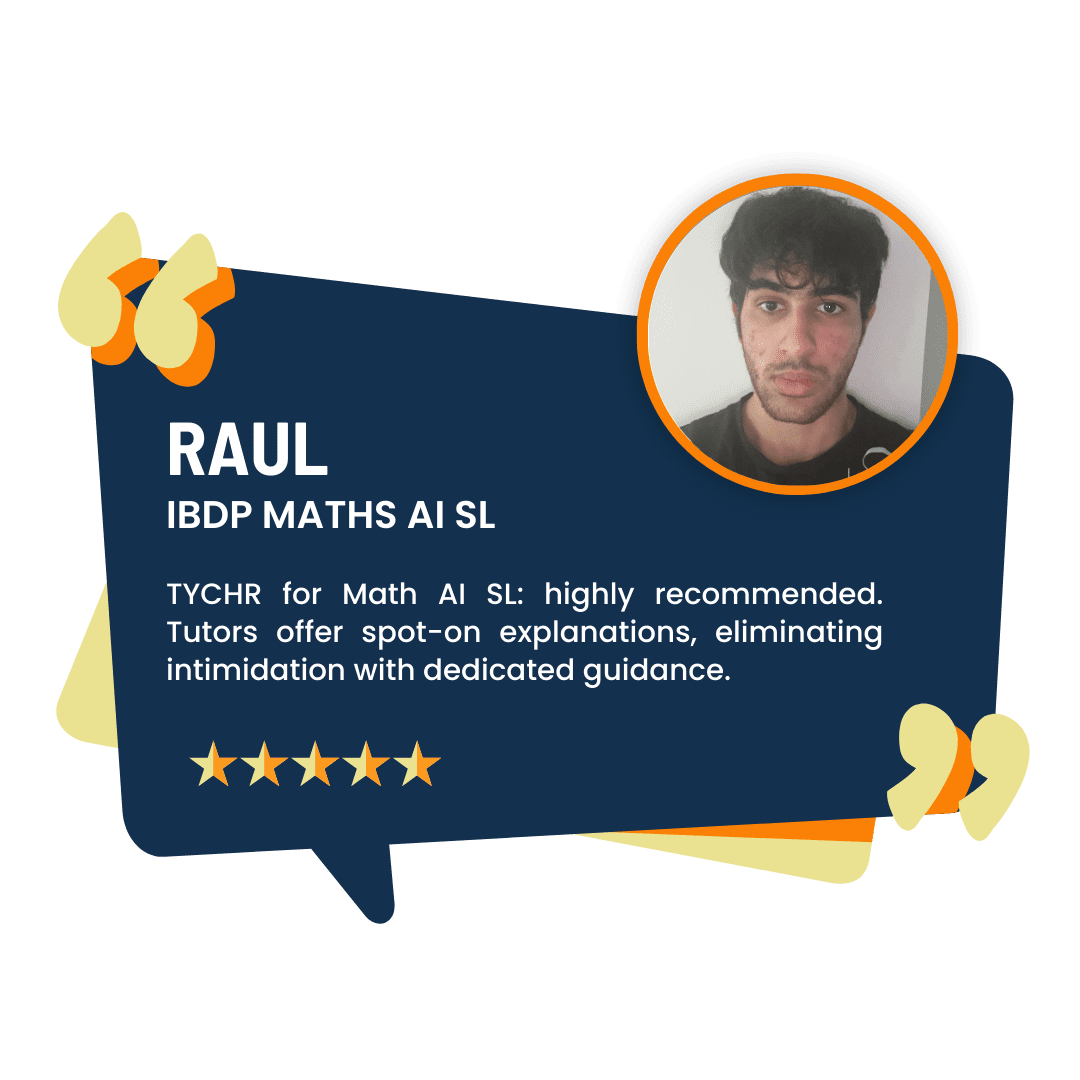
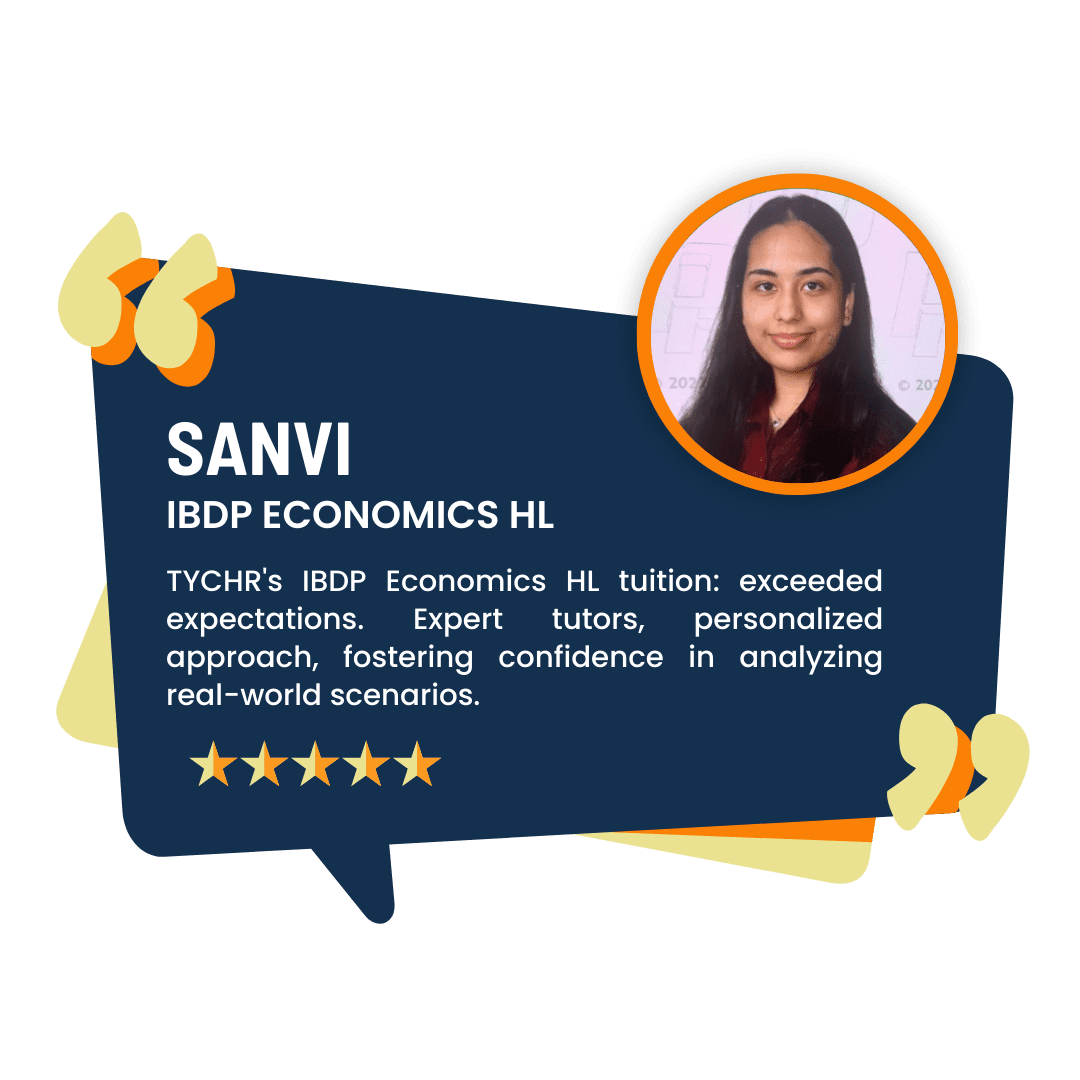
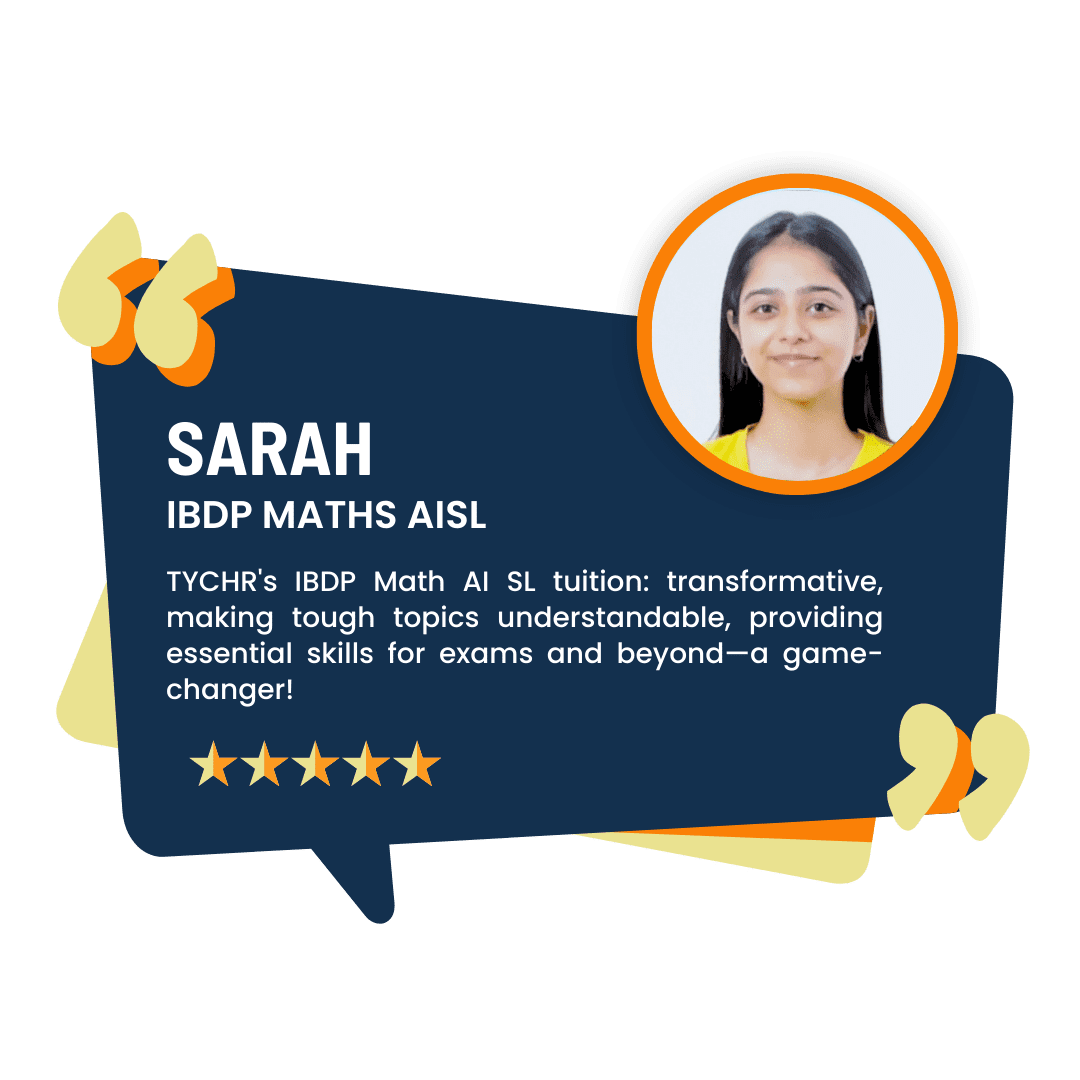
What our Students Have to say
Success stories from our students














FREQUENTLY ASKED QUESTIONS (FAQs)
A few frequently asked questions
Tychr provides support in international curricula,specialising in the IB. We have helped students across a diverse range of countries,not just India. With our proven methodology and expert tutors, we also provide exclusive resources developed by IB experts and offer one-on-one or group tuition, as well as university admission test support. Our team of highly professional Educators and Counselors have developed effective strategies to succeed in the IB, sharing their secrets, tips, and tricks with students.
At Tychr, we showcase utmost flexibility and understand that your child’s schedule can be crazy and dynamic. However, our tutors are often dependent on their sessions as their primary source of income, so if you need to cancel a lesson, we require at least 24 hour notice. If notice is not given more than twice, our tutors reserve the right to charge a cancellation fee equal to the hourly rate.
We pride ourselves on our speed and efficiency in setting up tuition. We can set up tuition within 24 hours.
At Tychr, we recommend at least two hours per subject each week.We have found that meeting at least twice a week develops more engagement with the child and helps them be more responsible towards allotted tasks. It has often improved the efficiency and results.
We offer exclusive, top-quality resources developed by IB experts. Our resources include IB subject-specific notes and study guides, exemplar exam responses,past year papers and high-scoring internal assessments. These resources are available for digital download and are provided to all enrolled students.
We offer flexible payment options like Bank transfer,Google Pay,NEFT,UPI Transfer etc. We offer package payments after a free tested session. Our minimum package is of 20 hours
Our tutors are professional IB Teachers and Examiners teaching across various reputable IB Schools in the country. Not only are they highly skilled communicators, but they also have their own proven strategies that they have tried and tested on their students over the years to score results of 40+
Our recent blogs
You’ll find your answers here, guaranteed
















































