atomic structure Notes
Atomic structure is a fundamental concept in IB DP Chemistry, forming the basis for understanding chemical behavior and reactions. This topic explores the composition of atoms, electron configurations, isotopes, and atomic theories that have evolved over time. Mastering atomic structure is essential for success in both Standard Level (SL) and Higher Level (HL) IB Chemistry, as it serves as a foundation for more advanced topics.
The nuclear atom
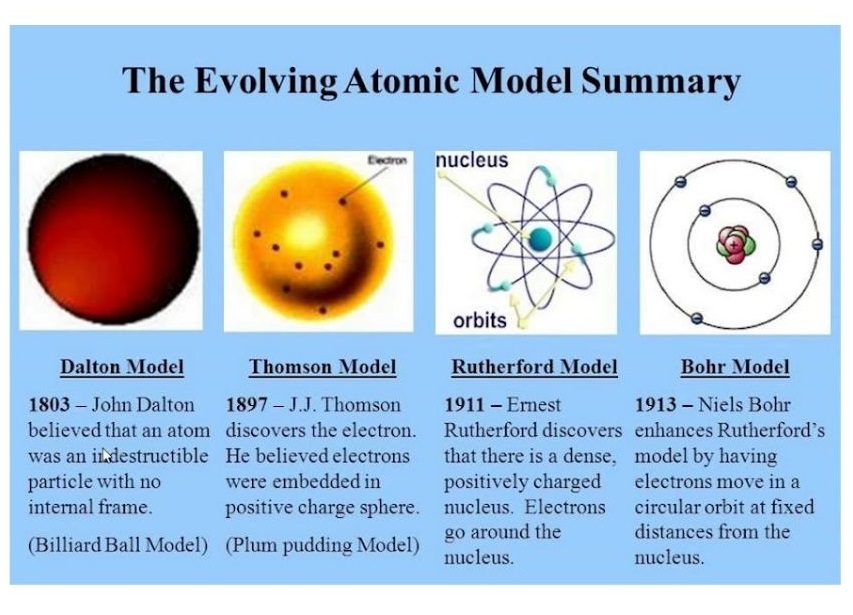
Dalton’s atomic theory
- Atom is the smallest unit of an element.
- Dalton’s theory can be summarized as follows:
1. All matter is composed of tiny indivisible particles called atoms;
2. Atoms can neither be created nor destroyed;
3. Atoms of the same element are alike in every way;
4. Atoms of different elements are different;
5. Atoms can combine together in small numbers to form molecules. - Compounds consist of atoms of more than one element and are formed by combining atoms in whole-number ratios.
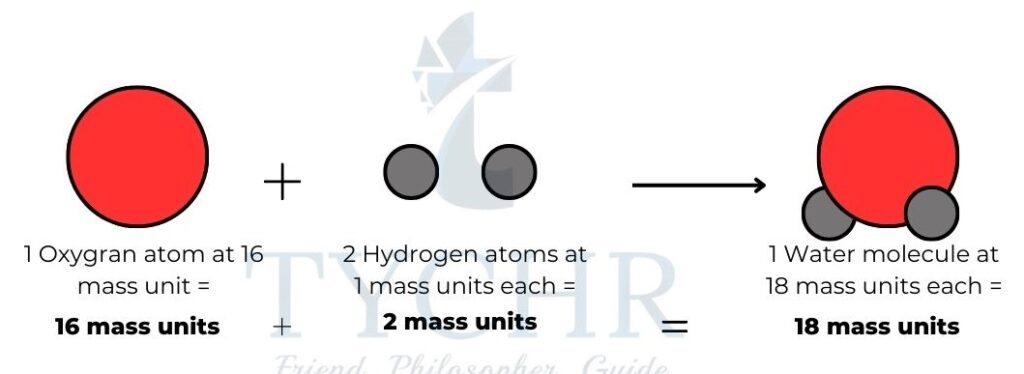
Figure 1: A model of a water molecule made from two hydrogen atoms and one oxygen atom.
Thomson’s “plum-pudding” model of the atom
- Thomson worked on cathode rays, and concluded that atoms contain very small negatively charged particles called electrons.
- In Thomson’s model, the atom is composed of electrons surrounded by a soup of positive charge to balance the electron (negative charges).
- Like negative charges “plums” surrounded by positively charged “pudding”.
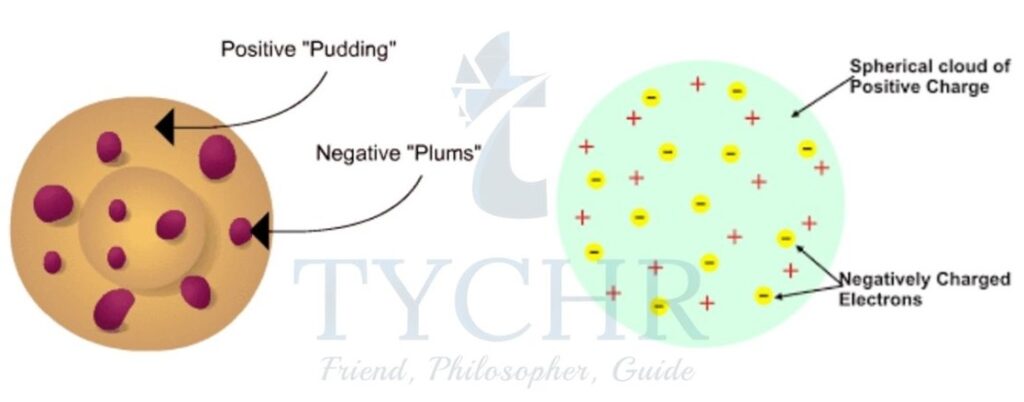
Figure 2: Thomson pictured the atom as a ‘plum pudding’, with the negatively charged electrons scattered in a positively charged sponge-like substance.
Rutherford’s model of the atom
- The alpha particles used for the experiment were helium nuclei, composed of two protons and two neutrons, with a positive charge.
- They are emitted by nuclei with too many protons to be stable.
- The large number of undeflected particles led to the conclusion that the atom is mainly empty space.
- Large deflections occur when the positively charged alpha particles collide with and are repelled by a dense, positively charged centre called the nucleus.
- The core of the atom lies a dense region of positive charge called the nucleus.
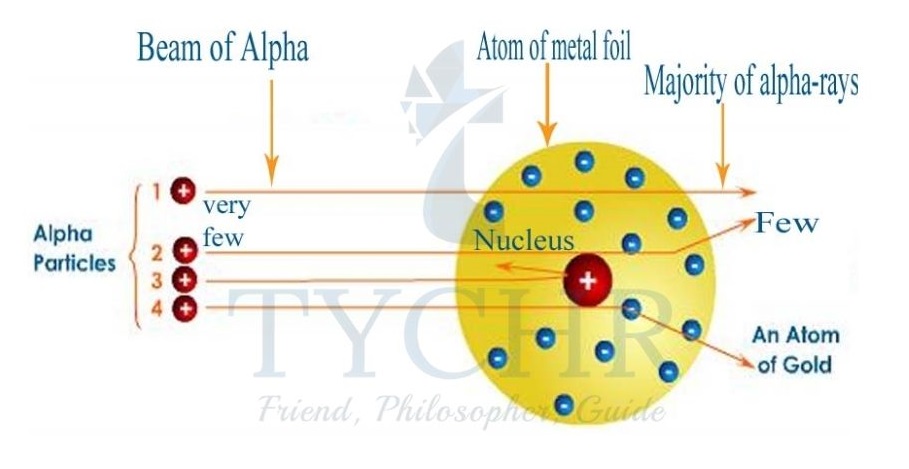
Figure 3: Rutherford’s model of the atom accounts for the experimental observations. Most of the alpha particles pass straight through but a small number collide with and are repelled by a positively charged nucleus.
Bohr model of the hydrogen atom
- Niels Bohr described that an atom is a positively charged nucleus, composed of protons and neutrons, surrounded by a negatively charged electron cloud.
- In the model, the electrons orbit the nucleus in atomic shells.
- The atoms are held together by electrostatic force of attraction between the positive nucleus and negative surroundings.
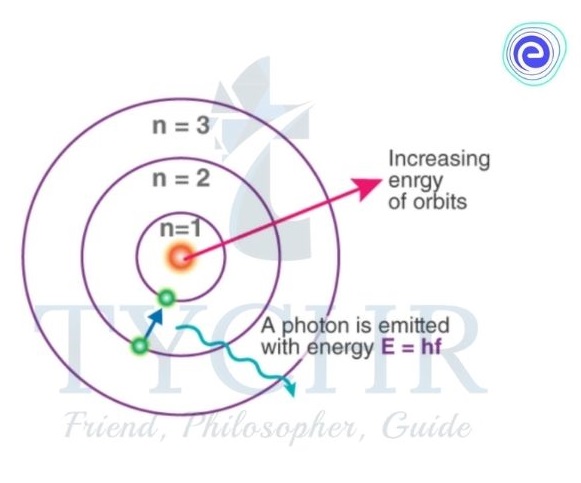
Figure 4: The Bohr model for hydrogen atom.
Summary
Subatomic particles and descriptions of the atom
- Atoms consist of three types of subatomic particles: the proton, the neutron and the electron.
- The atomic number (Z) is the number of protons in the nucleus of an atom of an element. Different elements have different atomic numbers.
- The mass number (A) is the number of protons + the number of neutrons in the nucleus of an atom.
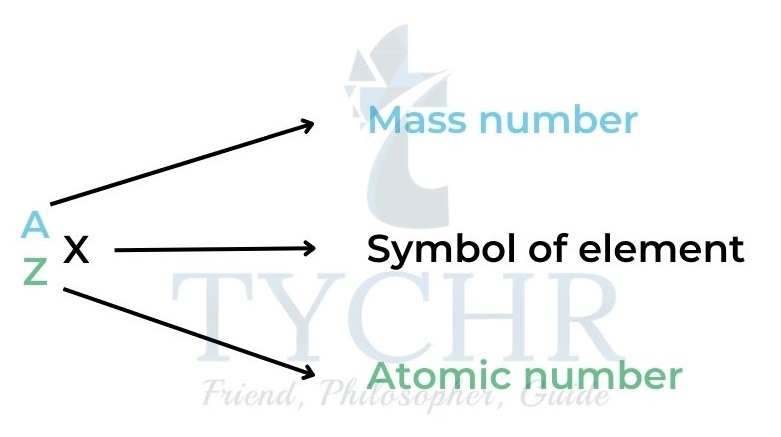
- We can use these numbers to find the composition of any atom. Number of protons (p) =number of electrons = Z Number of neutrons (n) =A – number of protons =A – Z
- Isotopes are different forms of the same element that have the same atomic number, Z, but different mass numbers, A, because they have different numbers of neutrons in their nuclei.
- The number of protons and neutrons never changes during a chemical reaction.
- Atoms can lose or gain electrons to form ions.
- When an atom loses electrons it forms a positive ion or cation, as the number of protons is now greater than the number of electrons.
- Negative ions or anions are formed when atoms gain electrons.
- The magnitude of the charge depends on the number of electrons lost or gained.
Relative atomic mass
- The mass of the atom is concentrated in the nucleus in the protons and neutrons.
- However, the mass of a single atom is tiny, and it is more convenient to use a system of
relative atomic masses. - The unified atomic mass unit is a non-SI unit of mass and is defined as one-twelfth of the mass of a carbon-12 atom in its ground-state.
- The relative atomic mass, Ar, is the ratio of the average mass of the atom to the unified atomic mass unit.
The mass spectrometer
- The mass spectrometer is an instrument used to determine the relative atomic mass of an element.
- There are five stages in this process:
- Stage 1 (vaporization): The sample is injected into the instrument where it is heated and vaporized, producing gaseous atoms or molecules.
- Stage 2 (ionization): The gaseous atoms are bombarded by high energy electrons, generating
positively charged species: X(g) + e → X+(g) + 2e - Stage 3 (acceleration): The positive ions are attracted to negatively charged plates and accelerated in the electric field.
- Stage 4 (deflection): The positive ions are deflected by a magnetic field perpendicular to their path. The degree of deflection depends on the mass-to-charge ratio (the m/z ratio). The species with the smallest mass, m, and the highest charge, z, will be defected the most. Particles with no charge are not defected in the magnetic field.
- Stage 5 (detection): The detector detects species of a particular m/z ratio. The ions hit the counter and an electrical signal is generated.
Figure 6: Schematic diagram of a mass spectrometer.
- The mass spectrum of gallium in Figure 7 shows that in a sample of 100 atoms, 60 have a mass of 69 and 40 have a mass of 71.We can use this information to calculate the relative atomic mass of the element.
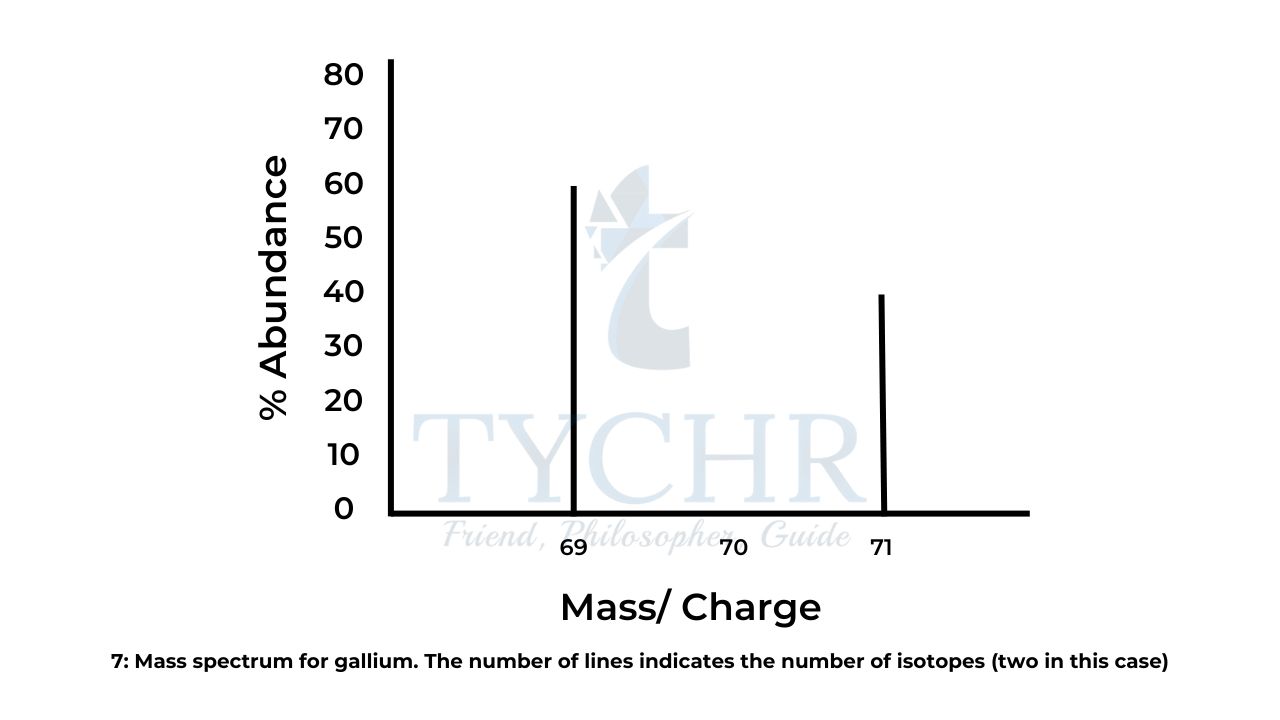
- Total mass of 100 atoms = (60 × 69) + (40 × 71) = 6980
total mass
Relative average mass = number of atoms = 6980/100 = 69.80
Electron configuration
The electromagnetic spectrum
- Electromagnetic radiation can be defined as a form of energy that is produced by the movement of electrically charged particles travelling through a matter or vacuum or by oscillating magnetic and electric disturbance.
- Examples of electromagnetic radiation include radio waves, visible light, microwaves, infrared radiation (IR), ultraviolet radiation (UV), X-rays, and gamma rays.
- The electromagnetic spectrum (EMS) is a spectrum of wavelengths that comprise the various types of electromagnetic radiation.
- The energy, E, of electromagnetic radiation is inversely proportional to the wavelength, λ:
E 𝖺 1/λ. - All electromagnetic waves travel at the same speed (c) but can be distinguished by their different wavelengths (λ).
- The number of waves which pass a particular point in 1 s is called the frequency ( ν ); the
shorter the wavelength, the higher the frequency. - High-energy radiations such as gamma rays and X-rays have small wavelengths, and low- energy radiations such as radio waves have long wavelengths.
- Wavelength is related to the frequency of the radiation, ν, by the expression: c = νλ
where c is the speed of light (3.00 × 108 ms-1). - The SI unit of energy is the joule, J; for wavelength the metre, m; and for frequency the hertz, Hz.
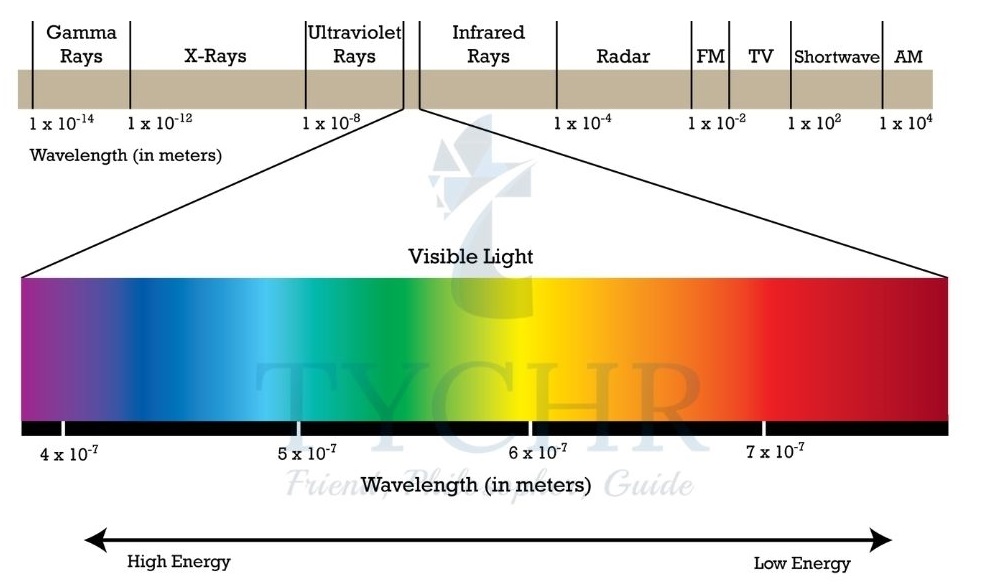
Figure 8: The changing wavelength (in m) of electromagnetic radiation through the spectrum is shown by the trace across the top.
Atomic absorption and emission line spectra
- White light is a mixture of light waves of differing wavelengths or colours. We see this when sunlight passes through a prism to produce a continuous spectrum or as a rainbow when light is scattered through water droplets in the air.
- When electromagnetic radiation is passed through a collection of atoms, some of the radiation is absorbed and used to excite the atoms from a lower energy level to a higher energy level. The spectrometer analyses the transmitted radiation relative to the incident radiation and an absorption spectrum is produced.
- The spectrometer analyses the transmitted radiation relative to the incident radiation and an absorption spectrum is produced.
- When white light is passed through hydrogen gas, an absorption line spectrum is produced with some colours of the continuous spectrum missing.
- If a high voltage is applied to the gas, a corresponding emission line spectrum is produced.
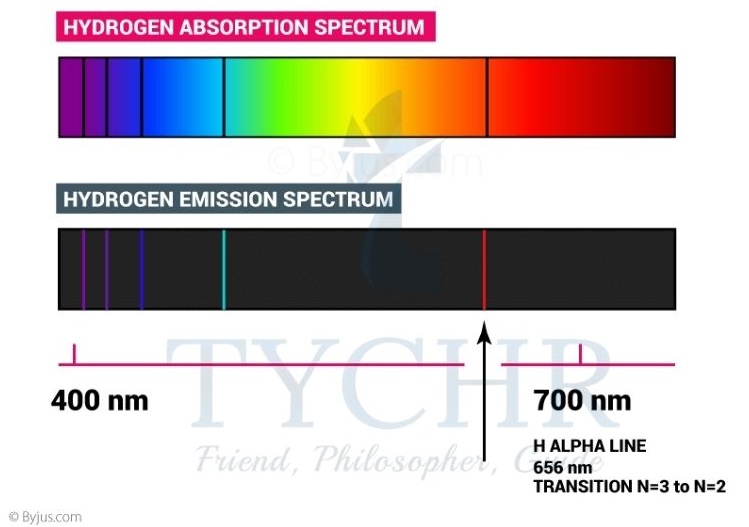
Figure 9: The origin of absorption and emission spectra. An absorption spectrum shows the radiation absorbed as atoms move from a lower to a higher energy level. An emission spectrum is produced when an atom moves from a higher to a lower level.
Evidence for the Bohr model
- Niels Bohr proposed that an electron moves into an orbit or higher energy level further from the nucleus when an atom absorbs energy.
- The excited state produced is, however, unstable and the electron soon falls back to the lowest level or ground state.
- The energy the electron gives out when it falls into lower levels is in the form of electromagnetic radiation.
- One packet of energy (quantum) or photon is released for each electron transition.
- The energy of the photon is proportional to the frequency of the radiation.
Ephoton = h ν - The atoms emit photons of certain energies which give lines of certain frequencies, because the electron can only occupy certain orbits.
- This energy of the atom is quantized.
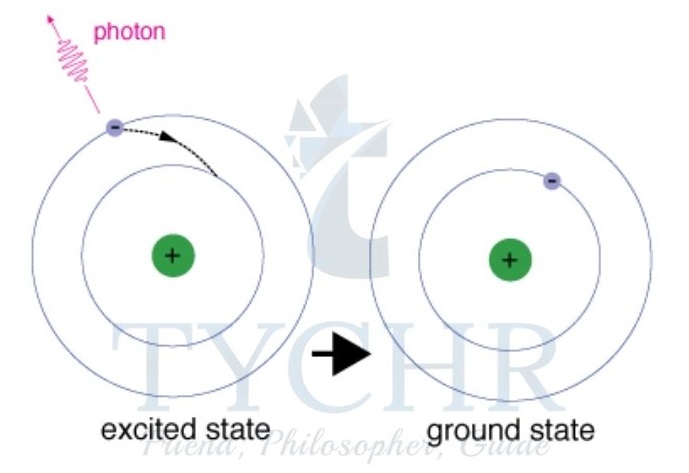
Figure 10: Emission and absorption spectra are the result of an energy transition between the ground and excited states.
The hydrogen spectrum
- The hydrogen line emission spectrum consists of a series of lines of different colours (violet, blue, blue–green, and red) in the visible region of the spectrum.
- The series of lines shown in figure 11 is called the Balmer series, which comprises lines associated with electronic transitions from upper energy levels back down to the n = 2 energy level.
- The pattern of the lines in Figure 11 gives us a picture of the energy levels in the atom
- The lines converge at higher energies because the energy levels inside the atoms are closer together at higher energy.
- When an electron is at the highest energy n = ∞, it is no longer in the atom and the atom has been ionized.
- The energy needed to remove an electron from the ground state of an atom in a mole of gaseous atoms, ions, or molecules is called the ionization energy.
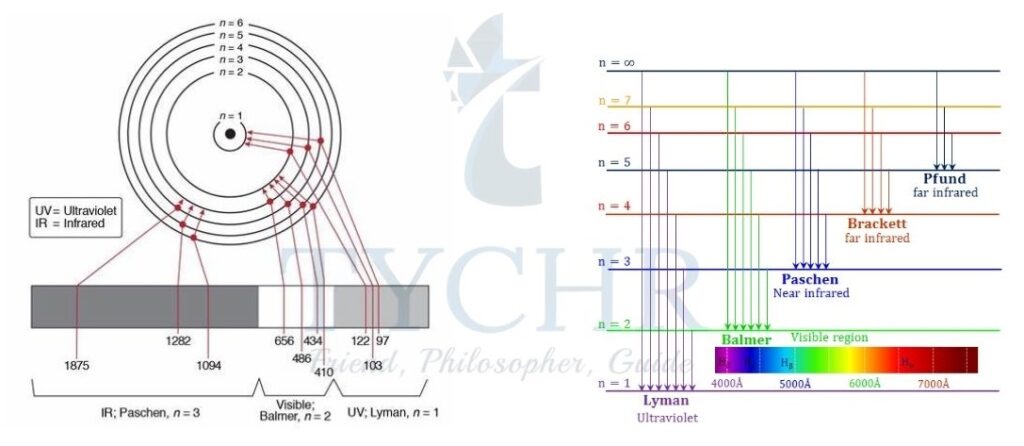
Figure 11: Energy levels of the hydrogen atom showing the transitions which produce the Lyman, Balmer, and Paschen series.
| Series | nf | ni | Region of EMS |
| Lyman | 1 | 2, 3, 4, 5…. | UV |
| Balmer | 2 | 3, 4, 5, 6…. | visible and UV |
| Paschen | 3 | 4, 5, 6, 7…. | IR |
The quantum mechanical model of the atom
- The wave function describes the electron in the hydrogen atom and associated possible energy states the electron can occupy.
- Each wave function is represented by the symbol,ψ. The square of the wavefunction, ψ2, represents the probability of finding an electron in a region of space at a given point a distance, r, from the nucleus of the atom. Ψ2 is termed the probability density.
- The wavefunctions of electrons in an atom are described by atomic orbitals: An atomic orbital is a region in space where there is a high probability of finding an electron.
- Any orbital can hold a maximum of two electrons. There are several types of atomic orbital: s, p, d, and f, etc. Each type has a characteristic shape and associated energy.
- An s orbital is spherically symmetrical. The sphere represents a boundary surface, meaning that within the sphere there is a 99% chance or probability of finding an electron. A p orbital is dumbbell shaped.
- The Bohr model introduced the idea of a main energy level, described by n, which is called the principal quantum number.
- The energies of the orbitals also increase as n increases. Each main energy level or shell can hold a maximum number of electrons given by 2n2.
- The energy levels are split up into sublevels, of which there are four common types: s, p, d, and f.
| Sublevel | Number of orbitals in Sublevel | maximum number of electrons in sublevel |
| s | 1 | 2 |
| p | 3 | 6 |
| d | 5 | 10 |
| f | 7 | 14 |
- For convenience, an “arrow-in-box” notation called an orbital diagram is used to represent the electrons in these atomic orbitals.
- Two electrons in the same orbital have opposite values of the spin magnetic quantum number, ms.
- There are three principles that must be followed when representing electron configurations.
1) The Aufbau principle states that electrons fill the lowest-energy orbital that is available first.
2) The Pauli exclusion principle states that any orbital can hold a maximum of two electrons, and these electrons have opposite spin.
3) Hund’s rule of maximum multiplicity states that when filling degenerate orbitals (orbitals of equal energy), electrons fill all the orbitals singly before occupying them in pairs.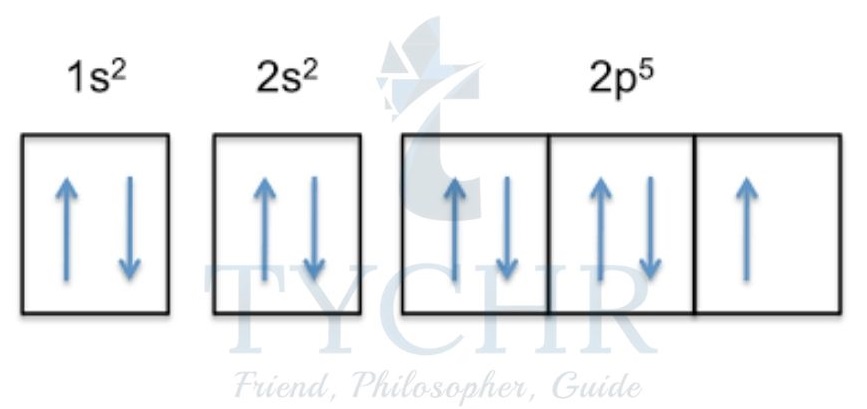
Figure 12: Electrons fill each orbital singly before occupying them in pairs.
Three ways electron configurations can be illustrated
(1) Full electron configuration
- The full electron configuration for some of the elements are given below.
- He: 1s2 , Ne: 1s22s22p6 , Ar: 1s22s22p63s23p6, Kr: 1s22s22p63s23p63d104s24p6
(2) Condensed electron configuration
- You can see above that full electron configurations become quite lengthy and cumbersome with increasing atomic number.
- An element’s chemistry is dictated by its outer valence electrons (as opposed to the inner core electrons), and a more convenient way of representing electron configurations is as the condensed electron configuration. ([nearest noble gas core] + valence electrons)
- For example: O: [He]2s22p4 , Ne: [He]2s22p6.
(3) Orbital diagrams
- Orbital diagrams make use of the arrows-in-boxes notation, with arrows representing electrons and boxes representing orbitals.
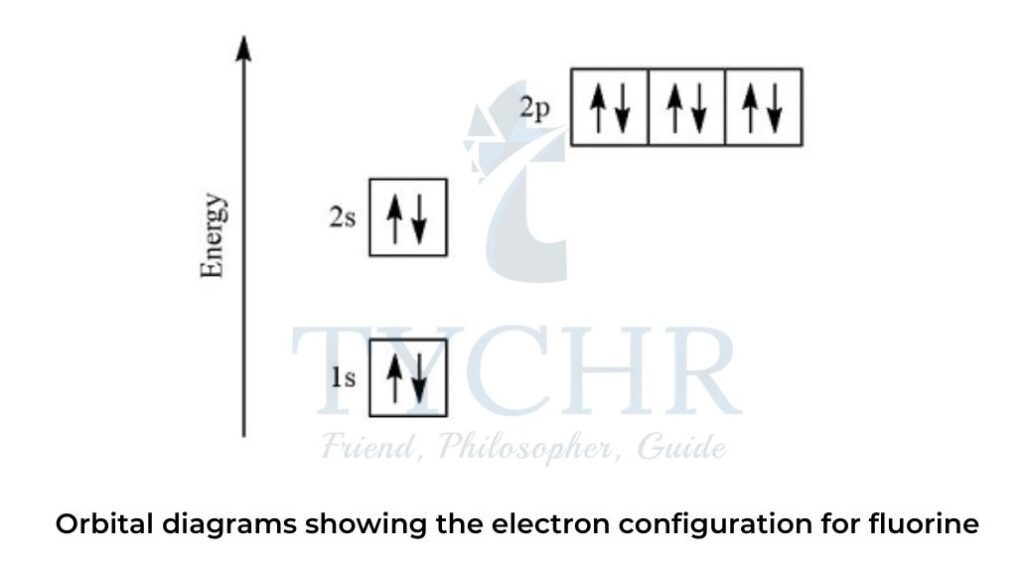
Electrons in atoms
Emission spectra and ionization
- Emission spectra provide experimental evidence for the existence of atomic energy levels.
- Ionization energy is defined as the minimum energy required to remove an electron from a neutral gaseous atom or molecule in its ground-state.
- The first ionization energy (IE1) of a gaseous atom is related to the process: X(g) → X+(g) + e
- Successive ionizations are also possible; for example, the second ionization energy (IE2) is associated with the process: X+(g) → X2+(g) + e.
- The nth ionization energy (IEn) relates to the process: X(n 1)+(g) → Xn+(g) + e
- The graph in Figure 14(a) shows two key points: (1) There is an increase in successive ionization energies. (2) There are jumps when electrons are removed from levels closer to the nucleus.
- The graph in figure 14 (b) shows the jump between the ninth and tenth ionization energies shows that the eleventh electron is more difficult to remove than we would expect from the pattern of the six previous electrons.
- This suggests that the second energy level is divided into two sub-levels.
- This evidence confirms that the 2s sub-level can hold a maximum of two electrons, and the 2p sub-level can hold six electrons.
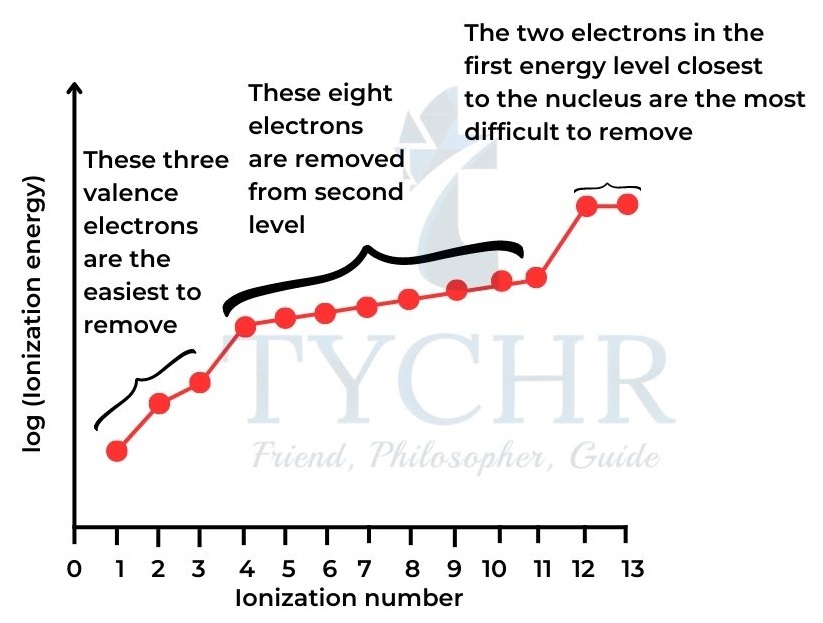
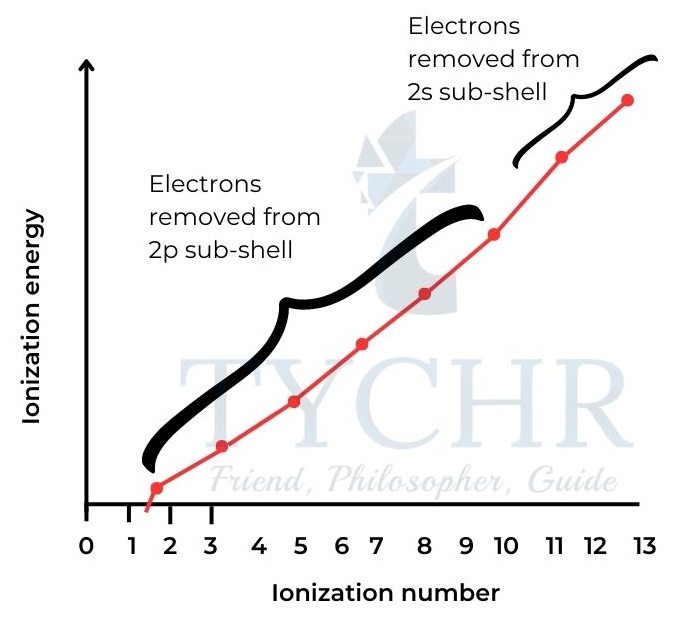
Figure 14: Successive ionization energies for aluminium.
Periodic trends in ionization energies
- The periodic arrangement of the elements in the Periodic Table is also reflected by patterns in first ionization energies.
(1) Ionization energy generally increases from left to right across a period, as the nuclear charge increases.
(2) Ionization energy decreases down a group as a new energy level, which is further from the nucleus, is occupied. Less energy is required to remove outer electrons that are further from the attractive pull of the nucleus.
(3) There are regular discontinuities in the trend across a period. These provide further evidence for the existence of sub-shells.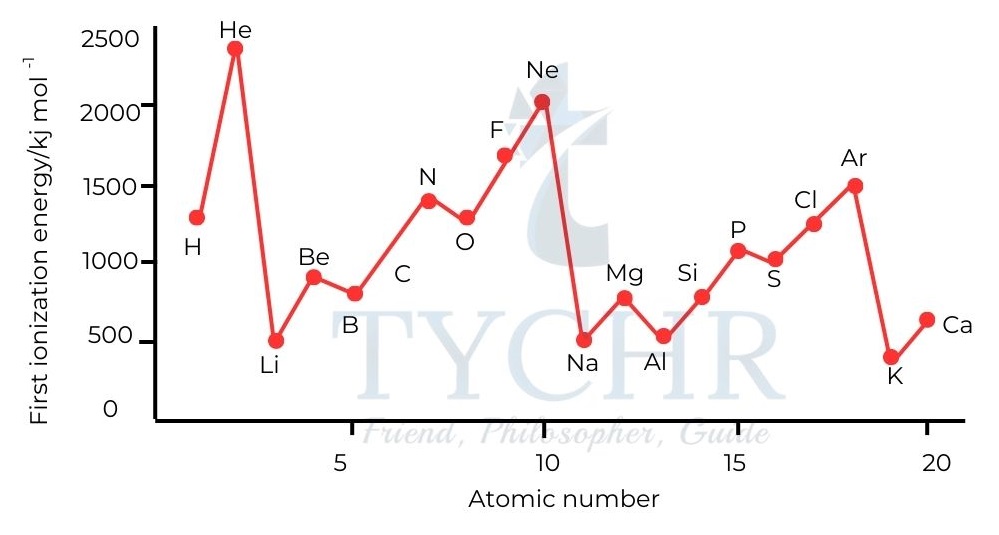
Figure 15: The first ionization energies of the first 20 elements.
A strong grasp of atomic structure is crucial for excelling in IB DP Chemistry, as it underpins many core concepts in the syllabus. Whether you’re studying SL or HL Chemistry, understanding atomic theory, electron configurations, and periodic trends can significantly enhance problem-solving skills. If you need extra support, Tychr.com offers expert IBDP Chemistry SL & HL tutors who provide personalized guidance to help students master the subject and achieve top grades.

