Energy production Theory Notes
Energy production is vital for modern society. This study note explores the physics behind various energy sources, crucial for the IBDP Physics syllabus. We’ll cover fossil fuels, nuclear power, and renewables (solar, wind, hydro), examining the physics principles of energy transformations, efficiency, and power. Understanding these foundations is key to analyzing energy resources and their impact.
Energy sources
Primary and secondary sources:
- A primary source is directly consumed without any transformation. Fossil fuels like coal, kinetic energy from water and air come under this category.
- Secondary source is obtained by transforming a primary fuel. Electical energy is obtained from internal energy of coal. Hydrogen is obtained by dissociation of water, hydrocarbons by using a primary source to supply required energy for dissociation.
Renewable and non-renewable energy sources:
Primary energy sources can be classified into renewable and non-renewable sources based on the rates at which they are formed and consumed.
- Renewable sources like biomass replenish themselves in short periods of time or have unlimited supply such as solar, wind or water. An advantage in using biomass is that the carbon trapped in biomass is recently taken from atmosphere.
- Non-renewable resources are consumed much faster compared to the rate at which they are formed. Fossil fuels are formed due to the effects of high temperature and pressure on vegetation for hundreds of millions of years. When these fuels are consumed, the carbon trapped in them at the time of formation is released causing increase in carbon dioxide concentrations in atmosphere.
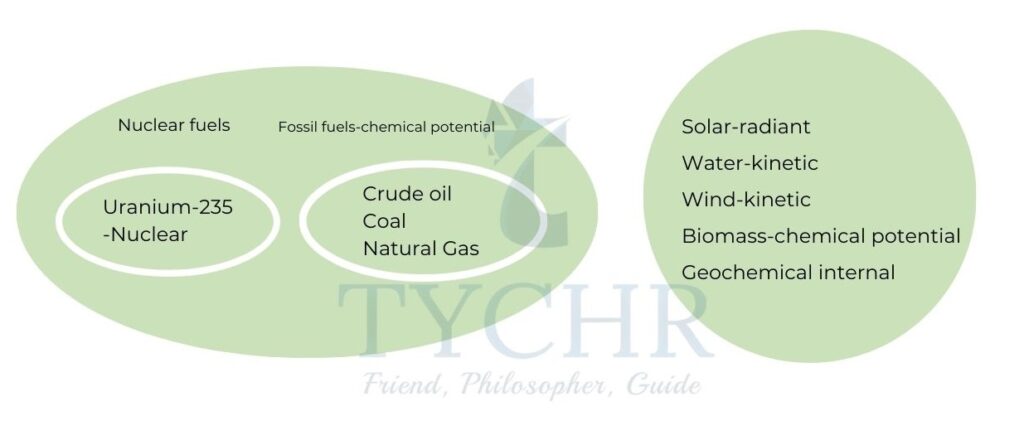
Energy sources:
The energy sources, their classification along with their form of energy through which they are consumed is given below.
Non-renewable sources
Commercial units of energy consumption:
- The British Thermal Unit (BTU) is the amount of energy used to raise the temperature of one British pound of water by 1 ˚F and is roughly about 1000J.
- One Million ton of oil equivalent (Mtoe) is the energy released when one ton of crude oil is burnt and is about 42GJ.
Specific energy and energy density:
- Specific energy is the energy that can be released by each kilogram of fuel.
E.g.: Find the mass of fuel consumed in a second in a fossil fuel power plant of 25% efficiency generating 1200MW of electrical energy. The specific energy of fuel is 52MJ/kg
Ans. With 25% efficiency, the energy to be supplied to the plant is (100/25) × 1200 = 4800MW
So, the mass of fuel required is 4800/52 = 92kg/s
- Energy density is the energy that can be released by 1m3 of fuel.
E.g.: A gasoline stove with 70% efficiency is used to heat water. The energy density of gasoline is 35GJ/m-3. What is the volume of gasoline required to raise the temperature of 1litre of water from 10˚C to 100˚C if the specific heat capacity of water is 4.2kJ kg-1 K-1.
Ans. The amount of heat required to raise the temperature of 1litre of water, which has a mass of 1 kg from 10 ˚C to 100 ˚C is 4200 × 1 × 90 = 0.38MJ
Energy required with 70% efficiency is 0.38/0.7 = 0.54MJ
So, volume of gasoline required is (0.54×106)/(35×109) =1.6× 10−4m3 or about 200ml.
Specific energy and Energy density of some fuels:
Fuel | Specific energy | Energy density |
Wood | 16 | 104 |
Coal | 20-60 | (20-60) × 106 |
Gasoline (petrol) | 45 | 35 × 106 |
Natural gas at atmospheric pressure | 55 | 3.5 × 104 |
Uranium (nuclear fission) | 8 × 107 | 1.5 × 1015 |
Deuterium/tritium (nuclear fusion) | 3 × 108 | 6 × 1015 |
Water falling through 100m in a hydro-electric plant | 10-3 | 103 |
It can be seen that nuclear sources clearly have high specific energy and energy density values and water, as a source of energy has least specific energy and energy density values.
Thermal power stations:
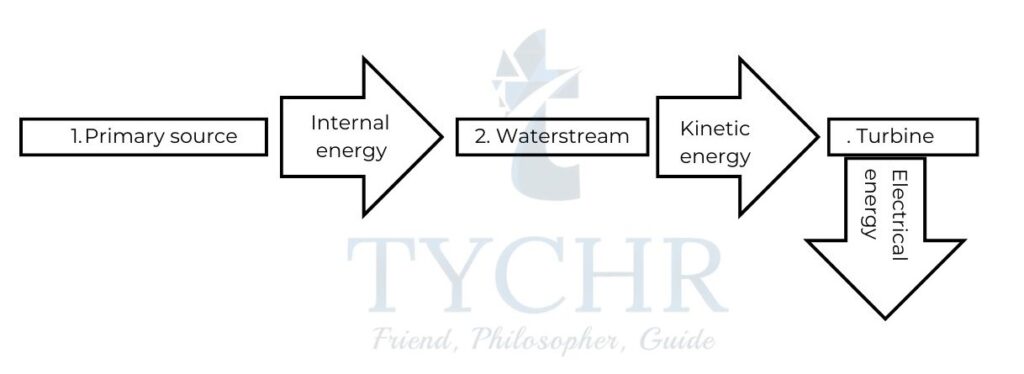
- The primary source of energy is first converted to internal energy. If the primary source is a fossil fuel or biomass, it is directly combusted. If it is a nuclear fuel then it involves a much more complicated process.
- The internal energy of primary source is used to convert water to steam contained in boilers at high pressures due to which the conversion to steam also takes place at temperatures well above normal boiling point of water. Steam at high pressure and temperature is directed to a set of turbines where it is converted to kinetic energy.
- The turbines are connected to a generator with a common axle. This generator converts the kinetic energy to electrical energy.
Sankey diagrams:
Sankey diagram is a diagrammatic representation of flow of energy in a device or a process.
Few rules to remember about Sankey diagrams:
- Each energy source and loss are represented by an arrow.
- The width of the arrow is proportional to the amount of energy it represents.
- The energy flow is drawn from left to right.
- When energy is lost, the arrow representing it is directed upwards or downwards.
E.g.: An electric kettle rated 2.0kW is switched on for 90s of which 20kJ is lost to surroundings. Represent the data in a Sankey diagram.
Ans. Energy supplied in 90s is kJ.
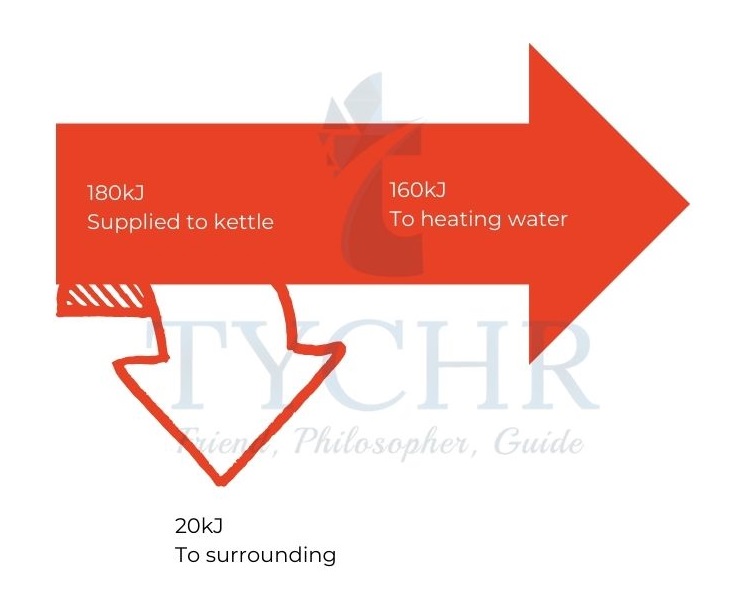
20kJ of 180kJ is 11%.
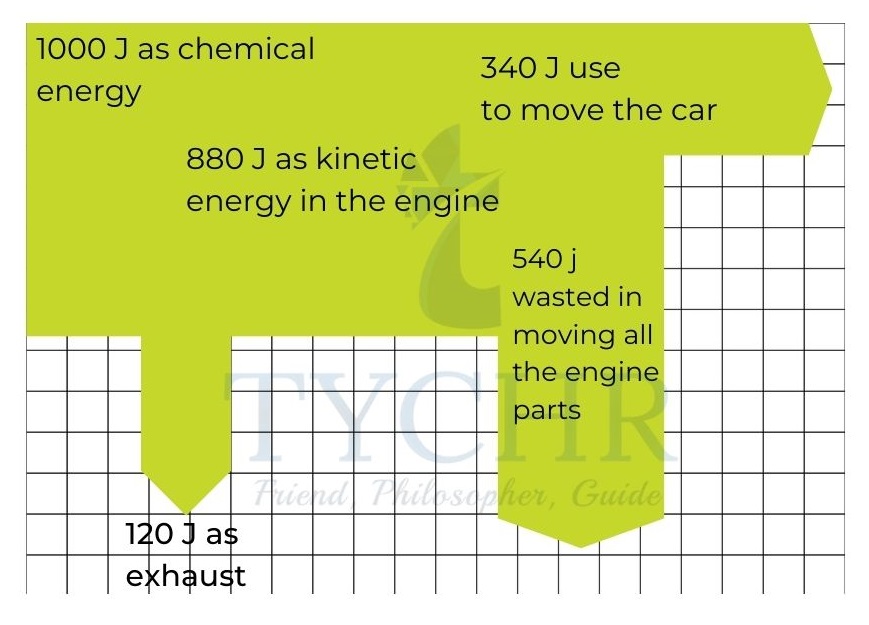
E.g.: In a petrol-powered car, 34% of the energy in fuel is converted into kinetic energy of car. 12% is lost through heated exhaust gases and the remaining energy is used in gearbox and wheels. Represent the data with a Sankey diagram.
Ans.
Primary sources used in power stations:
Fossil fuels:
The combustion process varies for each fossil fuel. While oil and gas can be readily burnt with a thermal connection to the boiler, coal has to be powdered and blown into the furnace.
Disadvantages of fossil fuels:
- Given the current rate of consumption, they will run out quickly and will not be replenished shortly.
- Burning the fossil fuels releases carbon dioxide trapped in them for millions of years. The greenhouse and enhanced greenhouse effect are a result of excessive release of greenhouse gases into the atmosphere
- Fossil fuels have significant use in fields of plastic, medicine and many others.
- Sometimes, fossil fuels have to be transported through large distances making the process inefficient.
Nuclear fuels:
Only nuclear fission is used as a source of energy. Fusion requires extreme temperatures and can’t be carried out with present technology. Pressurized water reactor (PWR) is a common nuclear reactor which uses uranium-235 as a nuclide. In few advanced gas-cooled reactors (AGR), carbon dioxide is used instead of water.
- When Uranium is extracted 99% of it is uranium-238. The ore has to be enriched to contain at least 3% uranium-235. The reason being uranium-238 a good absorber of neutrons and will prevent further fission reaction.
- The extracted uranium is made into fuel rods. The energy released and available for use will be in the form of kinetic energy of fission products and neutrons. When released these neutrons have a speed of about 104 kms-1.
- To stimulate further fission, the speed of neutrons should be about 2 kms-1. So, moderators in the form of water (sometimes heavy water-D2O) or graphite is used. A moderator should not be a good absorber of neutrons.
- Control rods are used to control or completely stop the power output in a reactor. These are materials of high neutron absorption capacity and are used to control the number of neutrons available for fission. When control rods fail to control the number of neutrons available for reaction, the reactor undergoes an uncontrollable reaction releasing large amounts of energy. This is called nuclear meltdown.
- The pressurized water transfers the energy from reactors to turbines in a way similar to fossil fuel station. The only difference being the heat exchange carried out in a closed system to prevent radioactive materials from getting transmitted outside.
E.g.: When one uranium-235 nucleus undergoes fusion, 3.2 × 10−11J of energy is released. The density of uranium-235 is 1.9 × 104 kgm-3. Calculate specific energy and energy density.
Ans. The mass of a uranium-235 atom is 1.7 × 10−27 × 235 = 4 × 10−25kg.
So, the specific energy is (3.2×10−11)/(
4.0×10−25) = 8 × 1013 Jkg-1.
1 kg of uranium-235 has a volume of 1/1.9 × 104 = 5 × 10−5 m-3.
So, energy density is (8.0×1013)/(5×10−5) = 1.6 × 1016 Jm-3.
E.g.: When a moving neutron collides head-on with a carbon-12 atom, it loses about 30% of its energy. Estimate the number of collisions required for an electron with 1MeV energy to be slowed down to 0.1eV.
Ans. After n collisions, the energy of the neutron will be 0.7𝑛 × 1 MeV. So,
0.7𝑛 × 1 MeV=0.1eV
n=45.2
i.e., it takes at least 46 collisions for the neutron to be sufficiently slowed down. In practice, it takes around 100 collisions, owing to the collisions being not head-on.
Safety measures in nuclear reactors:
- The reactor vessel is made of thick steel which in turn is enclosed in a thick layer of reinforced concrete. Concrete and steel absorb alpha, beta, gamma rays and stray neutrons.
- Fuel rods are operated by robots to avoid human contact.
Safety issues in nuclear reactors:
- The technology for proper disposal of radioactive waste and avoiding its contamination in food chain is still under development.
- After the life of a reactor, the reactor has to be decommissioned which involves removing the radioactive waste and enclosing the nuclear reactor and its enclosing in a larger shell of concrete and leave the structure alone for a century. This process is quite expensive and should be checked for economic feasibility.
Wind generators:
Wind generators consist of a rotor which rotates due to the wind flow against the blades of rotor connected through a gear box to electrical generator. The rotor can be mounted either horizontally or vertically and the design of blades changes accordingly.
The kinetic energy of air arriving at the turbine in one second can be estimated to be (1/2)ρAv3, where v is the velocity of wind, A is the area swept by the blades (πr2 if the radius of blades is r) and ρ is the density of air.
The electrical energy generated is a function of this kinetic energy determined by efficiency (𝜂) of the generator and other factors.
Wind generators are favored at off-shore locations where wind speeds are generally higher. Hills are also suitable for the same reason.
Merits and demerits:
Merits of wind generators include no chemical pollution, no energy costs and easy maintenance on land.
Demerits being no constant and reliable output, availability of land, noise, visual pollution and loss of habitat.
E.g.: Estimate the variation in maximum output power if :
- Wind speed doubles
- Radius of blade is halved
Ans. The maximum power output is (1/2)ρπr2v3. So, if the wind speed doubles, the power output increases 8 times, and if the blade radius is halved, it decreases 4 times.
E.g.: Given the density of air is 1.3kgm-3, blade length is 25m and wind speed is 11ms-1, estimate maximum power output from the wind generator.
Ans. The maximum power output is (1/2)ρπr2v3. Solving with given parameters, the maximum power output would be 1.7MW.
Pumped storage:
Water can be used to generate electricity in a number of ways like pumped storage plants, hydro-electric plants and wave energy. In pumped storage systems, the gravitational potential energy of water held in a reservoir at a height is converted to electrical energy. Water from higher reservoir is allowed to run when the demand for electricity is high and water can be pumped back to the reservoir when electricity is cheap.
Theoretically, the maximum electrical power output is (𝑽/𝒕)𝝆𝒈∆𝒉..
E.g.: Water from a pumped storage system falls through 260m at a rate of 600kgs-1. Find the electrical energy generated if the overall efficiency is 65%.
Ans. Electrical power generated is ɳ(𝑚/𝑡)gh.
Output powerr=0.65× 600 × 9.81 × 260 = 0.99MW.
E.g.: In a tidal barrage system water is retained behind a dam of height h. Show that the gravitational potential energy available from the water stored behind the dam is proportional to h2.
Ans. Assume that the cross-sectional area of the dam is A and that the cross-section is rectangular. The volume of water held by the dam is Ah. The mass of the water held by the dam is ρAh where ρ is the density of water.
When the dam empties completely the centre of mass of the water falls through a distance (h/2) (because the centre of mass is half way up the height of the dam).
The gravitational potential energy of the water is
mgh = (ρAh) × g ×h
Thus, gpe ∝ h2.
Solar energy:
Solar heating panels:
A pipe embedded under a black plate carries a mixture of water and glycol-glycol to prevent freezing of water in cold countries. The heated water is pumped to a heat exchanger containing a cylinder of water needed to be heated. A pump is required because the heated mixture is lighter and will stay in the pipe if it is not pumped. A control system is required to prevent pumping of colder water in case of cold conditions.
Solar photovoltaic panels:
The first photocells were developed in the middle of the 19th century by Alexandre-Edmund Becquerel. Photovoltaic cells are single crystal semiconductor panels doped such that one face is n-type and the other is p-type. When photons in the form of sun radiation fall on the panels, the equilibrium between the holes and electrons is disturbed and electric current flows through the external circuit.
Each cell has a potential of about 1V and many such cells are connected in a combination of series and parallel connection. The efficiency is around 20% and an increase is expected in future owing to the ongoing research.
Advantages are low maintenance costs, no fuel costs and no pollution of any form. Disadvantages are high initial costs and high inefficiencies.
Suppose the sun emits a radiation of intensity I and the area covered by the panels is A. then the power generated by photovoltaic panels of efficiency ɳ is ɳIA.
E.g.: A house requires an average power of 4.0kW. The intensity of sun radiation at the house is 650Wm-2. Calculate the minimum surface area of solar panels required to power the house if the efficiency of panels is 22%.
Ans. Area required is (4×1000)/(0.22×650) = 27.9 m2.
Thermal energy transfer
Thermal energy transfer:
1.) Thermal conduction:
Thermal energy is transferred through vibrations of particles. The atoms or molecules in regions of higher temperature vibrate with higher amplitude. These collide with molecules in regions of lower temperature and transfer the thermal energy until the whole body is of same temperature.
The rate of energy transfer through conduction along a bar of length L, area of cross-section A and the difference in temperatures at the ends is T is given by Δ𝑄/𝑡=𝐾𝐴Δ𝑇/𝐿 Here K is thermal conductivity of material.
Conduction is not effective in gases and solids due to large spacing between the molecules.
Effective heat transfer in gases and liquids takes place by another process called convection.
2.) Convection:
Convection occurs due to the difference in densities of a liquid at different temperatures. Consider a tank containing a liquid being heated at the bottom. The liquid at the bottom of tank gets heated and becomes less dense than its upper parts and rises. Whole liquid gets uniformly heated in the same way. This is called convection and the movement of liquid is called convection current.
Examples of convection:
- Sea breezes are formed due to uneven heating of land and sea. During the day, land is warmer and the air above it rises causing the air from sea to flow to land. During the night, sea is warmer and the reverse happens.
- Convection in earth is the cause of continental drift. The earth’s core is at a higher temperature and hence forces currents in upward direction causing continents to move apart.
3.) Radiation:
Radiation is the transfer of energy in the form of electromagnetic waves. Electromagnetic waves do not require a medium to travel. The energy from sun travels through radiation. Black bodies emit and absorb radiation better than white bodies.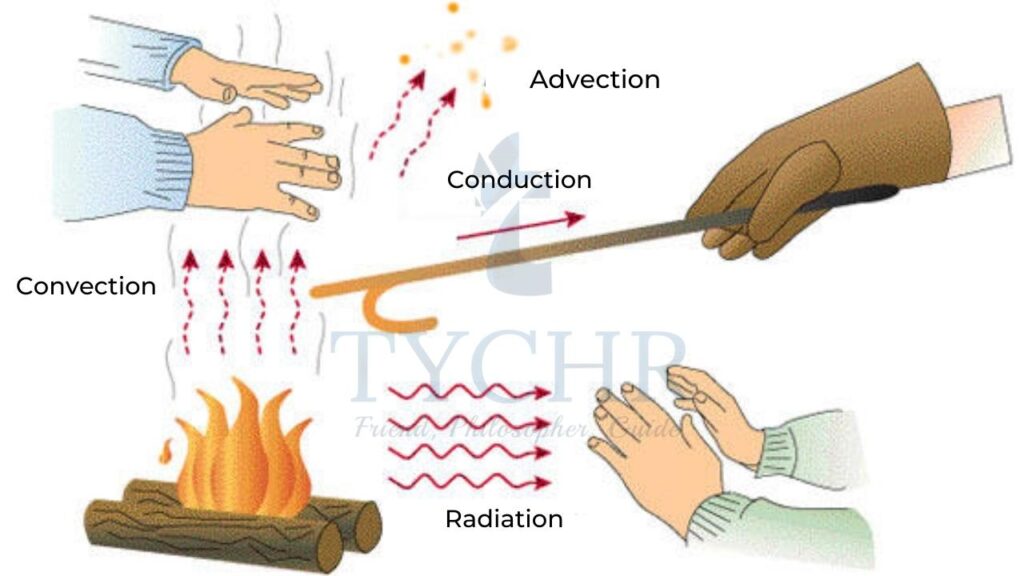
Black-body radiation:
Black-bodies are ideal bodies that absorb all the radiation incident on them. Black bodies emit radiation based on the temperature they are at. This radiation consists of a range of wavelengths with varying intensities.
This radiation follows the trend given below.
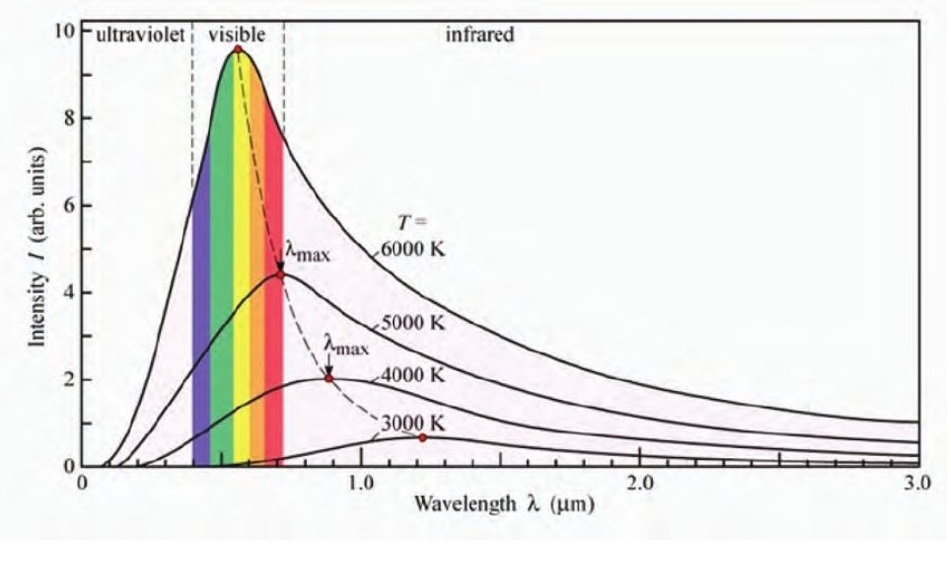
Wein’s displacement law:
The wavelength at which the intensity is maximum (𝜆𝑚𝑎𝑥) emitted by a black body at temperature (T) is given by
𝜆𝑚𝑎𝑥 = 𝑏𝑇
Here, b is called the Wein’s displacement constant and is given by 𝑏 = 2.9 × 103𝑚𝐾−1.
Stefan-Boltzmann law:
The total power emitted by a black body at a temperature T is given by
𝑃 = 𝜎𝐴𝑇4
A is the area of the black body and
𝜎 is the Stefan-Boltzmann constant given by 𝜎 = 5.7 × 10−8𝑊𝑚−2𝐾−4
Grey bodies and emissivity:
In real world, black bodies do not exist but bodies that behave close to black bodies called grey bodies exist. Emissivity of such bodies is given by:
𝑒 = 𝑝𝑜𝑤𝑒𝑟 𝑟𝑎𝑑𝑖𝑎𝑡𝑒𝑑 𝑏𝑦 𝑡ℎ𝑒 𝑔𝑟𝑒𝑦 𝑏𝑜𝑑𝑦/𝑝𝑜𝑤𝑒𝑟 𝑟𝑎𝑑𝑖𝑎𝑡𝑒𝑑 𝑏𝑦 𝑎 𝑏𝑙𝑎𝑐𝑘 𝑏𝑜𝑑𝑦 𝑜𝑓 𝑠𝑎𝑚𝑒 𝑑𝑖𝑚𝑒𝑛𝑠𝑖𝑜𝑛𝑠 𝑎𝑡 𝑠𝑎𝑚𝑒 𝑡𝑒𝑚𝑝𝑒𝑟𝑎𝑡𝑢𝑟𝑒
The power emitted by a grey body at temperature T is 𝑃 = 𝑒𝜎𝐴𝑇4.
E.g.: A spherical black body has an absolute temperature T and surface area A. The surroundings are at a temperature T1. Find the power emitted y the body.
Ans. The power emitted by the body is 𝜎𝐴𝑇4.
The power absorbed by the body is 𝜎𝐴𝑇14.
So, the net power emitted by the body is P = 𝜎𝐴(𝑇4-𝑇14).
Solar Constant:
Solar constant is the amount of solar radiation incident in one second on an imaginary plane placed perpendicular to the line joining centers of earth and sun placed at the center of the earth of whose area is a square meter. This is a unit of intensity and this intensity received varies from time to time.
The intensity of 1400Jm-2s-1 is defined as one solar constant.
Albedo:
The albedo of a surface is the ratio of energy reflected by it to the energy incident on it.
𝑎 = 𝑒𝑛𝑒𝑟𝑔𝑦 𝑟𝑒𝑓𝑙𝑒𝑐𝑡𝑒𝑑 𝑏𝑦 𝑎 𝑠𝑢𝑟𝑓𝑎𝑐𝑒 𝑖𝑛 𝑎 𝑔𝑖𝑣𝑒𝑛 𝑡𝑖𝑚𝑒/𝑒𝑛𝑒𝑟𝑔𝑦 𝑖𝑛𝑐𝑖𝑑𝑒𝑛𝑡 𝑜𝑛 𝑡ℎ𝑒 𝑠𝑢𝑟𝑓𝑎𝑐𝑒 𝑖𝑛 𝑡ℎ𝑒 𝑠𝑎𝑚𝑒 𝑡𝑖𝑚𝑒
The average albedo of earth’s surface over a year is 0.35.
The green house effect and temperature balance:
The green house effect is the absorption and emission of infrared and ultraviolet radiation by gases in atmosphere and leading to a higher temperature (290K) than that of moon (255K) which is almost at the same distance as that of earth from the sun.
The infrared and ultraviolet radiation from the sun is absorbed by green house gases (CO2, H2O, CH4 and N2O) and reemitted in all directions. Most of the visible part of sun radiation reaches the surface of the earth. Part of it is absorbed and part of it is reflected. The earth also emits radiation due to its finite temperature. The energy radiated and the energy absorbed exist in a state of dynamic equilibrium.
Global warming:
Global warming is the increase in average temperature of earth over years primarily due to human actions. The following actions attribute to global warming:
- Increase in solar constant
- Reduced albedo due to melting of polar ice caps
- Reduced emissivity due to greenhouse gases
- Enhanced greenhouse effect
The following measures can be taken to reduce greenhouse effect:
- Increasing efficiency of power production
- Replacing use of coal and petrol with natural gas
- Using excessive heat from power stations to heat homes
- Increased use of renewable and nuclear power sources
- Use of hybrid vehicles
- Capturing carbon dioxide and storage
Energy production depends on fundamental physics. This study note has covered key theoretical concepts for diverse energy sources. Applying these principles to real-world systems and evaluating different methods is essential. For personalized support in IB DP Physics, including energy production and other syllabus topics, consider a tutor. TYCHR connects students with experienced IB Physics HL & SL tutors for tailored help. A strong grasp of energy production theory is crucial for IB DP Physics success and informed discussions about energy.

