Table of Contents
Introduction to Electronegativity
Welcome to the electrifying world of chemical bonding! Have you ever wondered how atoms come together to form molecules? Or why certain elements are more inclined to steal electrons than others? Well, my curious friends, prepare to be enlightened as we dive into the fascinating realm of electronegativity.
In this blog post, we will unravel the mysteries behind the electronegativity chart and explore its significance in understanding chemical bonding. From its historical roots to practical real-world applications, we’ll cover it all. So fasten your seatbelts and get ready for a captivating journey through the electronegativity chart!
But wait… what exactly is electronegativity? Let’s start at square one before delving deeper. Electronegativity is a measure of an atom’s ability to attract and hold onto electrons when chemically bonded with another atom. Think of it as a cosmic tug-of-war game between atoms vying for those precious little charged particles.
Now that our introduction has sparked your interest (pun intended), let’s delve into the intriguing history behind the creation of the electronegativity chart!
The History of the Electronegativity Chart
The electronegativity chart has a rich and fascinating history that dates back to the early 20th century. It all began with the pioneering work of American chemist Linus Pauling, who is often referred to as the father of modern electronegativity.
In 1932, Pauling introduced the concept of electronegativity in his book “The Nature of Chemical Bond.” He defined electronegativity as the ability of an atom to attract electrons towards itself when forming chemical bonds. This groundbreaking idea revolutionized our understanding of chemical bonding and paved the way for further research in this field.
To quantify electronegativity values, Pauling devised a scale known as the Pauling Scale. On this scale, fluorine was assigned an arbitrary value of 4.0, which served as a reference point for comparing other elements’ relative electron-attracting abilities.
Over time, researchers expanded upon Pauling’s work by refining and expanding upon his scale. Today, we have various versions of electronegativity charts available that assign numerical values to different elements based on their electron affinity and other factors.
These charts have become invaluable tools for chemists worldwide in predicting how atoms will interact with one another during chemical reactions. They provide crucial insights into molecular structure formation and help explain why certain compounds are more stable than others.
The development and evolution of the electronegativity chart continue to be areas of active research today. Scientists strive to refine existing models and explore new ways to measure or calculate electronegativity accurately across different elements and compounds.
By delving into The History of the Electronegativity Chart, we gain a deeper appreciation for its significance in understanding chemical bonding patterns—a testament to human curiosity and scientific progress!
Also Read: Spit Dinosaur: A Fascinating Discovery from Prehistoric Times
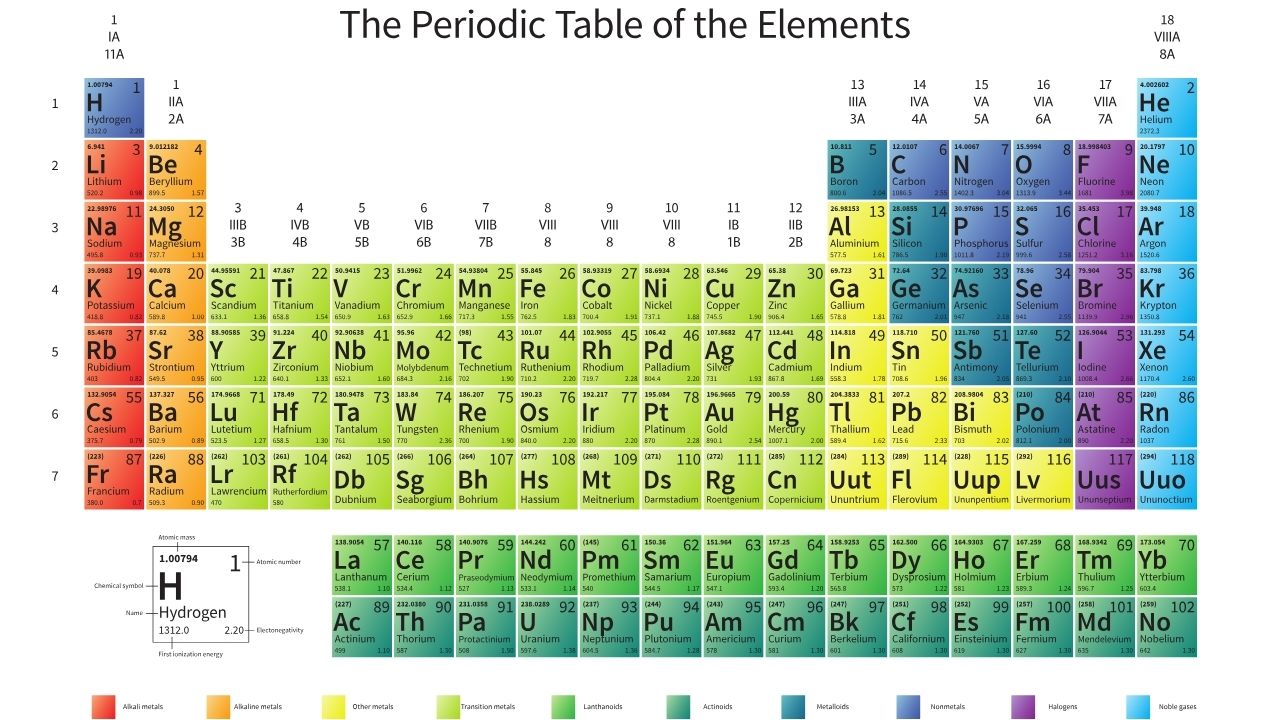
Explanation of the Periodic Table and its Relationship to Electronegativity
The Periodic Table is not just a colorful chart that you memorized in high school chemistry class. It’s actually a powerful tool that helps us understand the properties and behaviors of elements. And guess what? Electronegativity plays a crucial role in this!
You see, the Periodic Table is organized based on atomic number, which represents the number of protons in an atom’s nucleus. This arrangement allows us to observe trends and patterns in various chemical properties, including electronegativity.
Electronegativity is essentially a measure of an atom’s ability to attract electrons towards itself when it forms a chemical bond with another atom. It helps us determine how likely an element is to gain or lose electrons during bonding.
When we look at the Periodic Table, we can see that electronegativity generally increases from left to right across each period (horizontal row). This means that elements on the left side of the table tend to have lower electronegativities, while those on the right side have higher values.
Additionally, as we move down each group (vertical column) of the Periodic Table, electronegativity tends to decrease. So elements at the top have higher electronegativities compared to those at the bottom.
Understanding these relationships between electronegativity and position within the Periodic Table allows chemists and researchers to predict how different elements will interact and form bonds with one another. For example, if two atoms have significantly different electronegativities, they are likely to form an ionic bond where one atom transfers electrons completely or partially to another.
On the other hand, if two atoms have similar or close electronegativities, they are more likely to form covalent bonds where they share their electrons in order for both atoms to achieve stable electron configurations.
How to Read an Electronegativity Chart
Now that we understand the importance of electronegativity in chemical bonding, let’s dive into how to read an electronegativity chart. These charts are essential tools for scientists and chemists when studying elements and predicting their behavior in compounds.
An electronegativity chart is typically a table or graph that displays the electronegativity values of different elements. The most commonly used scale for measuring electronegativity is the Pauling scale, named after Linus Pauling, who made significant contributions to the field of chemistry.
The chart organizes elements according to their atomic number and groups them into periods and groups on the periodic table. Each element has a corresponding value assigned to it, which represents its relative attraction for electrons during chemical reactions.
To interpret an electronegativity chart, simply locate the element you are interested in and note its corresponding value. Higher values indicate stronger electron-attracting abilities, while lower values suggest weaker tendencies.
By comparing these values between two or more elements involved in a bond formation, you can determine what type of bond they will likely form – whether it be ionic (large difference), covalent (small difference), or polar covalent (moderate difference).
Remember that reading an electronegativity chart is just one part of understanding chemical bonding. It provides valuable insight into how atoms interact but should always be considered alongside other factors such as molecular geometry and electron configuration.
Knowing how to read an electronegativity chart allows us to predict and analyze chemical bonds more effectively. By understanding each element’s ability to attract electrons during reactions, scientists can make informed decisions about compound formation and reactivity patterns. So next time you come across an electronegativity chart, take a moment to explore its wealth of information!
Importance of Electronegativity in Chemical Bonding
Electronegativity plays a crucial role in chemical bonding, influencing how atoms interact and form compounds. It is the measure of an atom’s ability to attract electrons in a covalent bond. Understanding electronegativity helps us comprehend why certain elements are more likely to form specific types of bonds.
One key concept is the difference in electronegativity between two atoms involved in a bond. If there is a large difference, such as with sodium (Na) and chlorine (Cl), an ionic bond forms where electrons are transferred from one atom to another. This leads to the formation of charged ions that are attracted to each other.
On the other hand, if there is a smaller difference in electronegativity, like with hydrogen (H) and oxygen (O), a polar covalent bond occurs. In this type of bond, electron density shifts towards the more electronegative atom creating partial positive or negative charges.
The importance of electronegativity becomes evident when we consider chemical reactions and their outcomes. The variation in electronegativities among elements determines whether substances will dissolve easily or react readily with others.
Furthermore, understanding these principles allows scientists to predict molecular shapes and properties based on electron distribution patterns influenced by electronegativity differences within compounds.
Knowing about electronegativity helps explain why some molecules have distinct characteristics while others do not. By studying this fundamental concept, chemists can better understand how different elements combine and behave during chemical reactions leading to advancements across various fields including medicine, materials science, and environmental studies!
Also Read: NCAA Divisions: Understanding the Landscape of College Athletics
Real-World Applications of Electronegativity
Understanding electronegativity and its role in chemical bonding is not just a theoretical concept but has practical applications in various fields. Let’s explore some real-world applications of electronegativity:
1. Predicting Bond Type: Electronegativity helps scientists predict the type of bond that will form between two atoms. The difference in electronegativities can determine whether a bond will be ionic, covalent, or polar covalent. This knowledge is crucial for understanding the properties and behavior of different compounds.
2. Chemical Reactivity: Electronegativity also plays a vital role in determining the reactivity of elements and compounds. Elements with high electronegativities tend to be more reactive as they have a strong pull on electrons, making them more likely to participate in chemical reactions.
3. Solubility: The polarity of molecules affects their solubility in different solvents. By considering the electronegativities of atoms within a molecule, scientists can determine how soluble it will be in water or other solvents.
4. Biochemical Reactions: In biochemistry, understanding electronegativity is essential for studying enzyme-substrate interactions and protein folding processes. It helps explain why certain molecules bind together and how enzymes catalyze specific reactions within living organisms.
5. Material Science: Electronegativity influences material properties such as conductivity, magnetism, and optical behavior by influencing electron sharing or transfer between atoms within materials like metals or semiconductors.
6.Catalysis Studies: Catalysts play a crucial role in many industrial processes like petroleum refining and pharmaceutical production by increasing reaction rates without being consumed themselves fully.
Electronegativity values inform researchers about catalyst selectivity which depends upon charge distribution across reactants’ surface/active site.