IB Chemistry SL Paper 3 Question Bank
The IB Chemistry SL Paper 3 Question Bank is a great resource for students studying for the IB Chemistry SL exam. The Question Bank includes a wide variety of questions, all of which are relevant to the topics covered in the IB Chemistry SL course. The Question Bank is an excellent way for students to prepare for the IB Chemistry SL exam and get a feel for what kind of questions will be asked on the test.
Time: 1 hour
Instructions to candidates
- Answers must be written within the answer boxes provided.
- A calculator is required for this paper.
- A clean copy of the chemistry data booklet is required for this paper.
- The maximum mark for this examination paper is [35 marks].
SECTION A
1.) Physical properties and characteristics of elements vary according to the element’s atomic number.
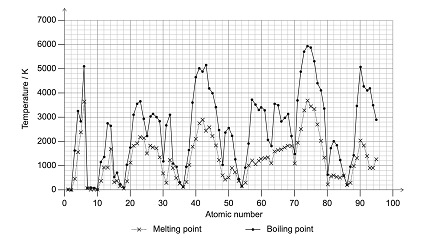
a.) Give a reason and state which group of elements are most likely to undergo sublimation
Answer:
Group 18 elements because the difference between the melting and boiling points are small. They also have the weakest intermolecular forces so they are more prone to undergo solid to gas phase change.
b.) Describe how the trend of the density varies across period 4 and 5
Answer:
The density increases to a maximum value and then begins to decrease
c.) Explain how the oxidation reaction differs for s-block and d-block metals with respect to their melting points and densities.
Answer:
S block elements with high densities and low melting points oxidize easier whereas, d block elements with low densities and high melting points oxidize easier.
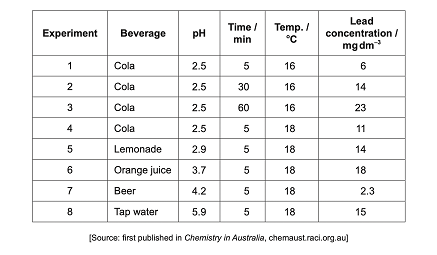
2.) There is a mug made of a lead alloy. The rate of lead dissolving in common beverages with various pH values was analyzed.
a.) The table below shows the data. Identify which experiment has the highest rate of lead dissolving.
Answer:
6 because the rate of lead dissolving is calculated by dividing lead concentration by time and experiment 6 gives the highest rate.
b.) Suggest why the relationship between the time and lead concentration for Cola at 16 degrees celsius is not linear.
Answer:
It is not linear because an equilibrium is being established between the lead in the solution and the alloy mug.
c.) Lead (II) Chloride has very low solubility in water
PbCl2 (s) ⇌ Pb2+ (aq) + 2Cl– (aq)
i.) Explain how the chloride ions in beverages affect the lead concentrations
Answer:
Equilibrium is shifted towards the reactants and the concentration of lead ions decreases as it precipitates.
SECTION B – Option A Materials
1.) Lithium has many applications
a.) State the bonding present in lithium hydride molecule and define it
Answer
Ionic bonding – It is when +ve (cations) and -ve (anions) ions are attracted to each other and form a continuous giant ionic lattice.
b.) Lithium is paramagnetic and Lithium hydride is diamagnetic. Outline why with reference to its electron configuration
Answer:
Paramagnetic properties are due to the presence of some unpaired electrons which Lithium has. However, all the electrons present in lithium hydride are paired.
2.) A catalyst is a substance that speeds up a chemical reaction without itself being consumed during the reaction.
a.) There are two kinds; heterogenous and homogenous. Differentiate between the two
Answer:
In a heterogeneous reaction, the catalyst is in a different phase from the reactants. In a homogeneous reaction, the catalyst is in the same phase as the reactants.
b.) Elastomers are used for the tyre treads. Explain how they can increase the traction between the tyre and the road.
Answer:
Elastomers tend to make tyres more flexible as they are loosely cross-linked polymers. The long randomly coiled materials can be stretched easily, but return to their original shapes when the force or stress is removed.
3.) The nematic liquid crystal phase is characterized by molecules that have no positional order but tend to point in the same direction.
a.) Describe the shape of the molecule and the effect that an electric field has on it.
Answer:
The shape of nematic liquid crystals are linear or a rod-like shape. When an electric field acts on the liquid crystal, the directional order increases i.e. the molecules arrange themselves in the same direction.
b.) State where nematic liquid crystals are widely used
Answer:
Liquid Crystal Displays (LCD’s)
c.) Nematics are a type of liquid crystals. Explain the effect of high and low temperatures on the liquid crystal behavior
Answer:
When it is in a very low temperature, the intermolecular forces prevent molecules from moving hence forming solid crystals. When it is in very high temperatures, it disrupts the alignment of the liquid crystal molecule and adapts to the characteristics of a fluid.
4.) Metals are usually extracted from their ores by a number of methods. Electrolysis and reduction with carbon are a few of them.
a.) Once the metal is extracted from its ore, the purity can be assessed using ICP Mass spectrometry. Suggest two advantages of using plasma technology in place of regular mass spectrometry.
Answer:
Plasma technology can detect multiple elements unlike mass spectrometry. They can also detect elements in low concentration.
b.) Outline how metals act as heterogeneous catalysts
Answer:
Reactants adsorb onto the active surface. Once they make contact, the bonds are weakened or broken. As this happens, the activation energy is lowered and the products are desorbed.
c.) Explain why alloys are harder than pure metals
Answer:
An alloy is made up of atoms of different sizes rather than being uniform like pure metals. The smaller or bigger atoms distort the layers of atoms in the pure metal. This means that a greater force is required for the layers to slide over each other. This makes the alloy harder and stronger than the pure metal.
d.) Carbon nanotubes can be formed from carbon monoxide. They’re used to increase the tensile strength of the metal. Write an equation to show the reaction
Answer:
2CO (g) → C (s) + CO2 (g)
Carbon nanotubes is a tube made of carbon with diameters typically measured in nanometers. Therefore, it explains the product of the reaction – Carbon.
5.) Polymers have a wide variety of applications but when it comes to disposing of them, they can be a problem.
a.) Define polymers
Answer:
A polymer is a long-chain molecule that is made up of many repeating units.
b.) A known polymer is the isotactic polychloroethene (PVC). Draw its monomer
Answer:
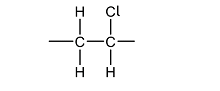
c.) Incinerating PVC can be harmful to the environment. State a product that is produced as a result of this.
Answer:
Carbon monoxide, Hydrochloric acid, Water, Carbon and dioxins
d.) Plasticizers affect the properties of polymers. Define plasticizers and outline its properties.
Answer:
A plasticizer is an additive that is added to another material to improve the polymers processability, flexibility, and stretchability by modifying the mechanical properties. Plasticizers are generally nonvolatile, high boiling and low molecular weight compounds.
OPTION B – Biochemistry
1.)
a.) Explain the role of ATP in metabolic reactions in cells.
Answer
ATP (adenosine triphosphate) is a molecule that carries energy within cells. It provides energy for metabolic processes such as biosynthesis, muscle contraction, and active transport. ATP is used as an energy source because it can easily release and store energy by breaking and forming phosphate bonds.
b.) State the equation for the hydrolysis of ATP to ADP and Pi.
Answer
ATP + H2O → ADP + Pi + energy
c.) Suggest two reasons why the initial rate of the reaction might increase at higher ATP concentrations.
Answer
The initial rate of the reaction might increase at higher ATP concentrations because there are more ATP molecules available to act as a substrate for the enzyme, and because ATP may act as an allosteric activator of the enzyme. Additionally, higher ATP concentrations may lead to a higher concentration gradient, which can increase the rate of diffusion of ATP to the enzyme active site.
2.)
a.) Define the term pH and explain how it affects enzyme activity.
Answer
pH is a measure of the concentration of hydrogen ions in a solution. It affects enzyme activity because enzymes have an optimum pH at which they function most effectively. Deviations from this optimum pH can denature the enzyme, altering its shape and preventing it from functioning properly.
b) State the equation for the dissociation of water and calculate the concentration of H+ ions in a solution with a pH of 5.
Answer
The equation for the dissociation of water is: H2O ⇌ H+ + OH–. In a solution with a pH of 5, the concentration of H+ ions is 10-5 mol/L.
c) A student investigated the effect of pH on the activity of the enzyme amylase. The student added amylase to a solution containing starch and measured the rate of starch hydrolysis at different pH values. The results are shown in the table below.
pH | Rate of starch hydrolysis (mg/min) |
5 | 0.5 |
6 | 2.5 |
7 | 5.0 |
8 | 3.2 |
9 | 1.0 |
Plot a graph of the results and explain the shape of the curve.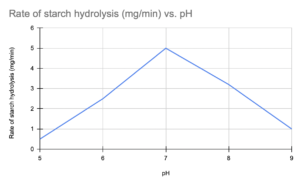
Answer
The graph of the results would show a bell-shaped curve, with the highest rate of starch hydrolysis at pH 7, the optimum pH for amylase. At pH values higher or lower than the optimum, the rate of starch hydrolysis decreases due to denaturation of the enzyme. At low pH, the excess H+ ions can disrupt the hydrogen bonds that hold the enzyme’s tertiary structure in place, and at high pH, the OH- ions can disrupt the ionic interactions between the amino acid side chains.
3.)
a.) Explain the difference between saturated and unsaturated fatty acids.
Answer
Saturated fatty acids contain only single covalent bonds between the carbon atoms in their hydrocarbon chains, while unsaturated fatty acids contain one or more double bonds between carbon atoms in their hydrocarbon chains.
b.) State the structural difference between cis and trans isomers of unsaturated fatty acids.
Answer
The structural difference between cis and trans isomers of unsaturated fatty acids lies in the arrangement of the atoms around the double bond. In cis isomers, the hydrogen atoms are on the same side of the double bond, whereas in trans isomers, they are on opposite sides.
c.) A case study describes a patient with high blood cholesterol levels. The patient’s blood was analyzed, and the results are shown in the table below.
Lipid | Concentration (mmol/L) |
Cholesterol | 7.5 |
Triglycerides | 2.1 |
HDL Cholesterol | 1.2 |
LDL Cholesterol | 4.8 |
i. Define the terms HDL and LDL cholesterol.
Answer
HDL (high-density lipoprotein) cholesterol is a type of cholesterol that is considered “good” because it helps to remove excess cholesterol from the bloodstream and transport it to the liver for processing. LDL (low-density lipoprotein) cholesterol is a type of cholesterol that is considered “bad” because it can build up in the walls of arteries, leading to atherosclerosis and an increased risk of cardiovascular disease.
ii. Explain the significance of the patient’s results in terms of their risk of cardiovascular disease.
Answer
The patient’s high blood cholesterol levels, particularly their high LDL cholesterol levels, indicate an increased risk of cardiovascular disease. This is because LDL cholesterol can build up in the walls of arteries and form plaque, which can narrow the arteries and reduce blood flow to the heart, leading to heart attacks and other cardiovascular problems. High levels of HDL cholesterol are generally considered protective against cardiovascular disease, but the patient’s HDL cholesterol levels are relatively low compared to their total cholesterol levels, which is also a risk factor for cardiovascular disease.
4.)
a.) Define the term enzyme and explain how enzymes affect the rate of a chemical reaction.
Answer
Enzymes are biological catalysts that increase the rate of a chemical reaction by lowering the activation energy required for the reaction to occur. Enzymes achieve this by binding to a specific substrate and forming an enzyme-substrate complex. The enzyme then catalyzes the conversion of the substrate to products, and is then released to catalyze another reaction.
b.) Describe the lock-and-key model of enzyme-substrate interactions.
Answer
The lock-and-key model of enzyme-substrate interactions describes the specific binding between an enzyme and its substrate. The active site of the enzyme is complementary in shape to the substrate, and this allows the substrate to fit into the active site like a key into a lock. Once the substrate is bound to the active site, the enzyme catalyzes the reaction to form products.
c.) A student investigated the effect of temperature on the activity of the enzyme catalase. The student added hydrogen peroxide to liver extract at different temperatures and measured the amount of oxygen produced. The results are shown in the table below.
Temperature (°C) | Oxygen produced (mL) |
20 | 0.5 |
30 | 3.4 |
40 | 5.7 |
50 | 5.5 |
60 | 2.1 |
Plot a graph of the results and explain the shape of the curve.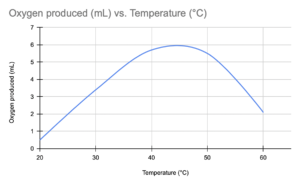
Answer
The graph should be plotted with temperature on the x-axis and oxygen produced on the y-axis. The shape of the curve will be a bell-shaped curve with a peak at the optimum temperature for catalase activity, which appears to be around 40°C in this case. The curve shows that the rate of the reaction increases with temperature up to a point, where it reaches a maximum and then decreases at higher temperatures due to enzyme denaturation.
OPTION C – Energy
1.) A student investigated the combustion of alcohols as a fuel. The following results were obtained.
Alcohol | Initial mass (g) | Final mass (g) | Temperature rise (°C) |
Methanol | 5.00 | 4.39 | 9.0 |
Ethanol | 5.00 | 4.10 | 11.5 |
Propanol | 5.00 | 4.00 | 7.2 |
a.) Calculate the heat energy released per gram of each alcohol burnt.
Answer
q = mcΔT
Methanol: q = (4.18 J g-1 K-1) (4.61 g) (9.0°C) = 169 J
Heat energy released per gram = 169 J / 5.00 g = 33.8 J g-1
Ethanol: q = (4.18 J g-1 K-1) (4.90 g) (11.5°C) = 236 J
Heat energy released per gram = 236 J / 5.00 g = 47.2 J g-1
Propanol: q = (4.18 J g-1K-1) (5.00 g) (7.2°C) = 151 J
Heat energy released per gram = 151 J / 5.00 g = 30.2 J g-1
b.) Which alcohol released the most heat energy per gram? Explain
Answer
Ethanol released the most heat energy per gram because it had the highest temperature rise and therefore the highest heat energy released.
c.) The complete combustion of methanol, ethanol, and propanol produces carbon dioxide and water. Write balanced chemical equations for the combustion of each alcohol.
Answer
Methanol: CH3OH(l) + 1.5O2(g) → CO2(g) + 2H2O(l)
Ethanol: C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l)
Propanol: C3H7OH(l) + 4.5O2(g) → 3CO2(g) + 4H2O(l)
2.) The diagram below shows the Born-Haber cycle for the formation of MgCl2.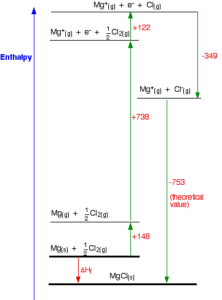
a.) Define the term lattice energy
Answer
The lattice energy is the energy required to separate one mole of a solid ionic compound into its gaseous ions.
b.) Explain why the lattice energy of MgCl2 is negative
Answer
The lattice energy of MgCl2 is negative because energy is released when the ions come together to form the solid.
c) Calculate the enthalpy change for the formation of MgCl2 using the Born-Haber cycle
Answer
ΔHf = ΔHsub + IE1 + EEA + ΔHLE – ΔHLat
ΔHsub = 148 kJ mol-1
IE1 = 738 kJ mol-1
EEA = -349 kJ mol-1
ΔHLE = 0 kJ mol-1
ΔHLat = -2522 kJ mol-1
ΔHf = (148 + 738 – 349 + 0 – 2522) kJ mol-1 = -1945 k
d.) Explain why the experimental value for the enthalpy change of the reaction may differ from the calculated value
Answer
The experimental value for the enthalpy change of the reaction may differ from the calculated value due to experimental errors such as heat loss to the surroundings or incomplete reactions. The value calculated using the Born-Haber cycle assumes that all of the reactions are complete and that there are no energy losses.
3.) Graphite is a form of carbon with a layered structure. It is used as a lubricant due to its ability to easily slide over layers. The structure of graphite is shown below: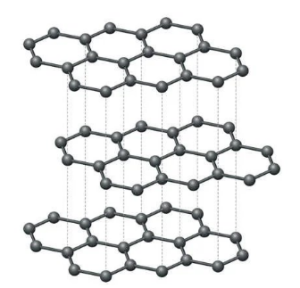
a.) Explain the bonding in graphite.
Answer
The bonding in graphite is covalent and involves sp2 hybridization of carbon atoms. Each carbon atom forms three covalent bonds with three neighboring carbon atoms, forming a hexagonal network of carbon atoms. The remaining electron forms a π bond that extends over a large number of atoms in a flat sheet, giving rise to the layered structure of graphite. This type of bonding is classified as a covalent network solid.
b.) Describe the layered structure of graphite
Answer
The layered structure of graphite consists of a series of parallel layers of carbon atoms arranged in a hexagonal lattice. Each carbon atom is bonded covalently to three neighboring carbon atoms, forming a planar hexagonal network. Adjacent layers are held together by weak van der Waals forces, resulting in a relatively weak interlayer interaction. The layers can slide over one another easily, allowing graphite to act as a good lubricant.
c.) Explain why graphite is a good lubricant
Answer
Graphite is a good lubricant due to its layered structure and weak interlayer forces. The layers can slide over one another easily, allowing graphite to act as a solid lubricant. The weak interlayer forces also mean that the layers can be easily sheared apart, reducing friction and wear between sliding surfaces.
d.) Compare the properties of diamond and graphite
Answer
Diamond and graphite are both allotropes of carbon, but they have vastly different properties due to their different structures. Diamond has a three-dimensional covalent network structure in which each carbon atom is bonded to four neighboring carbon atoms in a tetrahedral arrangement. This gives diamond its exceptional hardness and high melting point. Graphite, on the other hand, has a layered structure with weak interlayer forces. This gives graphite its ability to easily slide over layers and act as a good lubricant. Graphite is also much softer and has a lower melting point than diamond.
4.) The efficiency of a heat engine is given by the equation: efficiency = (T1 – T2) / T1 where T1 is the absolute temperature of the hot reservoir and T2 is the absolute temperature of the cold reservoir.
a.) Explain why the efficiency of a heat engine is less than 100%
Answer
The efficiency of a heat engine is less than 100% because some of the heat energy is always lost to the surroundings as waste heat.
b.) State two factors that can affect the efficiency of a heat engine
Answer
Two factors that can affect the efficiency of a heat engine are the temperature of the hot reservoir and the temperature of the cold reservoir.
c.) A heat engine operates between temperatures of 600 K and 300 K. Calculate the efficiency of the engine.
Answer
efficiency = (T1 – T2) / T1
efficiency = (600 K – 300 K) / 600 K = 0.50 or 50%
OPTION D – Medicinal Chemistry
1.)
a.) Define the term pharmacophore
Answer
A pharmacophore is the part of a molecule that is responsible for its biological activity, and includes the atoms and functional groups involved in binding to a target receptor or enzyme.
b.) Draw the structure of a molecule that could act as an opioid receptor antagonist. Label the functional groups responsible for the pharmacological activity of the molecule.
Answer
Structure:
O=C(CN)NC1=C(C(=O)N(CCN(CC)CC)C1=O)C
Functional groups: The carbonyl group (C=O) and the tertiary amine (N(CCN(CC)CC)).
2.)
a.) Define the term bioavailability
Answer
Bioavailability is the fraction of a drug that reaches the systemic circulation after administration, and is available to exert its pharmacological effects.
b.) A patient is prescribed a drug that has a bioavailability of 60%. If the dose is 500 mg, how much of the drug actually reaches the systemic circulation?
Answer
Amount of drug that reaches the systemic circulation = 500 mg x 0.60 = 300 mg
c.) Describe one factor that can affect the bioavailability of a drug
Answer
One factor that can affect the bioavailability of a drug is the route of administration. For example, oral drugs must pass through the gastrointestinal tract and liver before reaching the systemic circulation, which can lead to significant first-pass metabolism and reduced bioavailability.
d.) Suggest one strategy to increase the bioavailability of a poorly soluble drug.
Answer
One strategy to increase the bioavailability of a poorly soluble drug is to formulate it in a more soluble form, such as a salt or a prodrug. For example, the prodrug of the antihypertensive agent enalapril is a more water-soluble ester, which is rapidly converted to the active drug in vivo, leading to improved bioavailability.
3.)
a.) Define the term partition coefficient
Answer
Partition coefficient is a measure of the relative solubility of a compound in two immiscible phases, typically expressed as the ratio of the concentrations of the compound in each phase at equilibrium.
b.) The partition coefficient of a drug between octanol and water is 10. What does this value indicate about the drug’s solubility and lipophilicity?
Answer
A partition coefficient of 10 indicates that the drug is more soluble in octanol than in water, and has a higher lipophilicity (ability to dissolve in lipids) than hydrophilicity (ability to dissolve in water).
4.)
a.) Analyze the chemical structure of the commonly used antimalarial drugs, chloroquine and artemisinin, in terms of their mechanism of action and effectiveness.
i. Outline the structure and function of the Plasmodium falciparum parasite in causing malaria.
The Plasmodium falciparum parasite is a protozoan parasite that infects red blood cells and causes the symptoms of malaria. It has a complex life cycle that involves several stages of development, including invasion, replication, and release of new parasites.
ii. Compare and contrast the mechanisms of action of chloroquine and artemisinin.
Chloroquine is a quinoline derivative that works by interfering with the digestion of hemoglobin in the parasite, leading to the accumulation of toxic heme and ultimately killing the parasite. Artemisinin is a sesquiterpene lactone that reacts with iron to form reactive oxygen species that can damage the parasite’s membranes and other cellular components, leading to its death.
5.)
a.)
i. Outline the structure and function of the endocannabinoid system in the human body.
The endocannabinoid system is a complex signaling network that involves the binding of endogenous cannabinoids (such as anandamide and 2-arachidonoylglycerol) to cannabinoid receptors (CB1 and CB2) in the body. The endocannabinoid system is involved in a variety of physiological processes, including pain, mood, appetite, and immune function.
ii. Compare and contrast the pharmacological properties of tetrahydrocannabinol (THC) and cannabidiol (CBD).
THC is a psychoactive compound that binds to CB1 receptors in the brain and causes euphoria, relaxation, and altered perception. CBD, on the other hand, has little affinity for CB1 and CB2 receptors, and does not produce the same psychoactive effects as THC.
References:
Our Expert Tutors!
- 12+ Years
Cat 1 – ESS and Cat 2 – Biology. Chief of the IB program. Mentored 320+ students across various curricula.
- 24+ Years
IBDP Physics HL / SL. IGCSE Physics. A-level Physics (AQA, CIE, Edexcel, OCR, and WJEC). IGCSE Physics (AQA,CIE, OCR & Edexcel)
- 9+ Years
IBDP Cat 1 – Business Management, IBDP Cat 1 – TOK. Taught over 130+ students across 4+ countries.
- 9+ Years
IBDP Cat 1 & 2 November 2019. Specializes in Global Politics. Many students scored 7s; mentors 200+ students in assessments.
- 16+ Years
Specializing in Mathematics: Analysis and Approaches (HL & SL), Mathematics: Applications and Interpretation (HL & SL), and MYP (Mathematics).
- 18 + Years
IBDP Cat 1 – Chemistry, IBDP Cat 3 – IA Chemistry, IBDP Cat 1 – TOK. Helped 2 out of 3 students achieve a 7 in IB Chemistry.