Table of Contents
- 1 Unit 1: Stoichiometric Relationships
- 2 Unit 2: Atomic Structure
- 3 Unit 3: Periodicity
- 4 Unit 4: Chemical Bonding and Structure
- 5 Unit 5: Energetics and Thermochemistry
- 6 Unit 6: Chemical Kinetics
- 7 Unit 7: Equilibrium
- 8 Unit 8: Acids and Bases
- 9 Unit 9: Redox Processes
- 10 Unit 10: Organic Chemistry
- 11 Unit 11: Measurement and data processing and analysis
- 12 Unit 12: Atomic Structure
- 13 Unit 13: Periodicity
- 14 Unit 14: Chemical Bonding and Structure
- 15 Unit 15: Energetics and Thermochemistry
- 16 Unit 16: Chemical Kinetics
- 17 Unit 17: Equilibrium
- 18 Unit 18: Acids and Bases
- 19 Unit 19: Redox Processes
- 20 Unit 20: Organic Chemistry
- 21 Unit 21: Measurement and Analysis
- 22 OPTION A: Materials
- 23 Option B: Biochemistry
- 24 Option C: Energy
- 25 Option D: Medicinal Chemistry
- 26 Frequently Asked Questions (FAQs)
- 26.1 Q1: What topics are covered in the IB Chemistry syllabus?
- 26.2 Q2: How is the IB Chemistry course assessed?
- 26.3 Q3: What skills do students need to succeed in IB Chemistry?
- 26.4 Q4: How is the IB Chemistry course different from other high school chemistry courses?
- 26.5 Q5: What are the benefits of taking the IB Chemistry course?
Unit 1: Stoichiometric Relationships
| Subtopic | Subtopic Number | IB Points to Understand |
| Introduction to the particular nature of matter and chemical change | 1.1 (SL) | Matter is defined as anything that has mass and takes up space (it has volume).
Properties of three states of matter (Solids, Liquids and Gasses) Temperature is a measure of the average kinetic energy of the particles of a substance Sublimation: When solid turns into a gas without first becoming liquid. Condensation: When gaseous state changes into liquid state. Melting and boiling are endothermic processes Condensation and freezing are exothermic processes Elements, Compounds and Mixtures An ion is a charge species Atom economy is the efficiency of chemical reactions by comparing the molecular mass of atoms in the reactants with the molecular mass of useful compounds |
| The Mole Concept | 1.2 (SL) | The mole is an SI unit, symbol mol, defined as a fixed amount, n, of a substance
The relative atomic mass Ar of an atom is a weighted average of the atomic masses of its isotopes and their relative abundances The empirical formula of a compound is the simplest whole-number ratio of atoms or amount (in mol) of each element present in a compound. The molecular formula is the actual number of atoms or amount (in mol) of elements in one structural unit or one mole of the compound, respectively. |
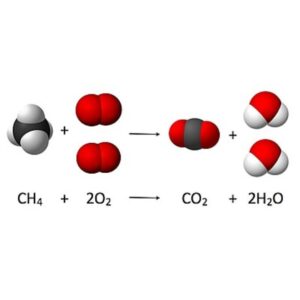
| Reacting masses and volumes | 1.3 (SL) | Chemical change is an expression of reactants combining in fixed ratios to form products.
The reactant that determines the quantity of product is known as the limiting reactant. The theoretical yield refers to the maximum amount of product obtainable, assuming 100% of the limiting reactant is converted to product. The kinetic theory of gasses is a model used to explain and predict the behavior of gasses at a microscopic level Standard temperature (0 °C/273 K) and Standard pressure (100kPa) Boyle’s Law: Relationship between volume and pressure Charles’s Law: Relationship between volume and temperature Gay-Lussac’s Law: Relationship between pressure and temperature Ideal Gas Equation: PV = nRT A solution is a homogenous mixture of a solute that has been dissolved in a solvent. Solute is usually a solid but it can be a gas or liquid too. |
Unit 2: Atomic Structure
| Subtopic | Subtopic Number | IB Points to Understand |
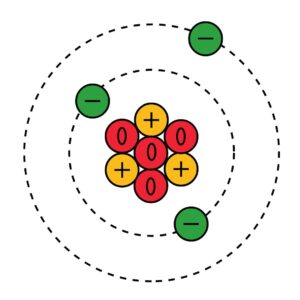
| The nuclear atom | 2.1 (SL) | Dalton’s Atomic Theory
Compounds consist of atoms of more than one element and are formed by combining atoms in whole-number ratios Atom is the smallest unit of an element Thomson’s Plum Pudding model of the atom Rutherford’s model of the atom Bohr Model of the Hydrogen atom Atoms consist of three types of subatomic particles: the proton, the neutron and the electron. The atomic number (Z) is the number of protons in the nucleus of an atom of an element. The mass number (A) is the number of protons + the number of neutrons in the nucleus of an atom. Isotopes are different forms of the same element that have the same atomic number, Z, but different mass numbers, A When atoms lose electrons, it is a cation. If atoms gain electrons, it becomes an anion. The unified atomic mass unit is a non-SI unit of mass and is defined as one-twelfth of the mass of a carbon-12 atom in its ground-state The mass spectrometer is an instrument used to determine the relative atomic mass of an element. |
| Electron configuration | 2.2 (SL) | Electromagnetic radiation: form of energy that is produced by the movement of electrically charged particles traveling through a matter or vacuum or by oscillating magnetic and electric disturbance.
The electromagnetic spectrum (EMS) is a spectrum of wavelengths that comprise the various types of electromagnetic radiation. All electromagnetic waves travel at the same speed (c) but can be distinguished by their different wavelengths (λ) The SI unit of energy is the joule, J; for wavelength the meter, m; and for frequency the hertz, Hz. When white light is passed through hydrogen gas, an absorption line spectrum is produced with some colors of the continuous spectrum missing. If a high voltage is applied to the gas, a corresponding emission line spectrum is produced The hydrogen line emission spectrum consists of a series of lines of different colors in the visible region of the spectrum. The energy needed to remove an electron from the ground state of an atom in a mole of gaseous atoms, ions, or molecules is called the ionization energy Ways to illustrate electron configurations |
Unit 3: Periodicity

| Subtopic | Subtopic Number | IB Points to Understand |
| The periodic table | 3.1 (SL) | Vertical columns: Groups
The Horizontal rows of elements numbered from 1 to 7 are termed period Period number is equal to the principal quantum number, n Properties of metals S, p, d, f and transition elements |
| Periodic trends | 3.2 (SL) | Effective nuclear charge
Atomic radius is the distance from the center of the nucleus to the outermost shell containing electrons The formation of positive ions involves the loss of the outer shell. The formation of negative ions involves the addition of electrons into the outer shell. The ionization energy, IE, is the minimum energy required to remove an electron from a neutral gaseous atom in its ground-state The first electron affinity of an element (∆Hea) is the energy change when one mole of electrons is added to one mole of gaseous atoms to form one mole of gaseous ions The Group 17 elements have incomplete outer energy levels and the Group 1 metals have the lowest effective nuclear charge Electronegativity, symbol χ, is defined as the relative attraction that an atom has for the shared pair of electrons in a covalent bond Group 1, 17 and 18 chemical properties |
Unit 4: Chemical Bonding and Structure
| Subtopic | Subtopic Number | IB Points to Understand |
| Ionic Bonding and Structure | 4.1 (SL) | Electrons positioned outside the nucleus, are less tightly held and outer electrons, known as valence electrons, can be transferred when atoms react together
An ionic Bond refers to the electrostatic attraction experienced between the electric charges of a cation (positive ion) and an anion (negative ion) Electrostatic forces: The oppositely charged ions resulting from electron transfer are attracted to each other and are held together by this force Ionic compounds: These forces are known as an ionic bond, and ions held together in this way The term coordination number is used to express the number of ions that surround a given ion in the lattice Physical properties of ionic compounds |
| Covalent Bonding | 4.2 (SL) | Covalent Bonding: atoms share electrons with each other in order to attain a noble gas electron configuration
In a Lewis symbol representation, each element is surrounded by a number of dots (or crosses), which represent the valence electrons of the element Bond strength and bond length |
| Covalent structures | 4.3 (SL) | Bonding pairs of electrons (showing the covalent bond as single, double, or triple bonds) and
Non-bonding pairs of electrons, often called the lone pairs, which are pairs of electrons not involved in the bonding Valence shell electron pair repulsion (VSEPR) theory can be used to deduce the shapes of covalent molecules Molecules with two electron domains will position them at 180° to each other Molecules with three electron domains will position them at 120° to each other Molecules with four electron domains will position them at 109.5° to each other Resonance involves using two or more Lewis structures to represent a particular molecule or ion Difference between graphite, diamond, fullerene and graphene |
| Intermolecular forces | 4.4 (SL) | Weak forces of attraction, known as London (dispersion) forces, will occur between opposite ends of these two temporary dipoles in the molecules.
Dipole Dipole attraction When a molecule contains hydrogen covalently bonded to a very electronegative atom (fluorine, nitrogen, or oxygen), they are attracted to each other by a hydrogen bond |
| Metallic Bonding | 4.5 (SL) | Delocalized: when there is no other element present to accept the electrons and form an ionic compound, the outer electrons are held only loosely by the metal atom’s nucleus
An alloy is a mixture that consists either of two or more metals, or of a metal (or metals) combined with an alloying element composed of one or more nonmetals |
Unit 5: Energetics and Thermochemistry

| Subtopic | Subtopic Number | IB Points to Understand |
| Measuring energy changes | 5.1 (SL) | Thermodynamics is the study of energy and how it is interconverted
The first law of thermodynamics states that energy can be converted from one form to another and that the total amount of energy for a given system will remain constant Enthalpy (H) is a measure of the amount of heat energy contained in a substance. It is stored in the chemical bonds and intermolecular forces as potential energy Exothermic reactions give out heat and result in a transfer of enthalpy from the chemicals to the surroundings and ∆H reaction is negative A few reactions are endothermic as they result in an energy transfer from the surroundings to the system. In this case the products have more enthalpy than the reactants and ∆H is positive The standard enthalpy change of combustion (∆H⊖c) is the enthalpy change for the complete combustion of one mole of a substance in its standard state in excess oxygen under standard conditions The standard enthalpy change of formation, ∆H⊖f, of a substance is the enthalpy change that occurs when one mole of the substance is formed from its elements in their standard states. |
| Hess’s Law | 5.2 (SL) | Hess’s Law states that the enthalpy change for a chemical reaction is independent of the route taken. |
| Bond Enthalpies | 5.3 (SL) | The bond enthalpy is the energy needed to break one mole of bonds in gaseous molecules under standard condition. |
Unit 6: Chemical Kinetics

| Subtopic | Subtopic Number | IB Points to Understand |
| Collision theory and rates of reaction | 6.1 (SL) | The rate of a reaction depends on how quickly the concentration of either reactant or product changes with respect to time.A colorimeter or spectrophotometer works by passing light of a selected wavelength through the solution being studied and measures the intensity of the light transmitted by the reaction componentThe total electrical conductivity of a solution depends on the total concentration of its ions and on their chargeMaxwell-Boltzmann distribution curveIn order for a collision to lead to reaction, the particles must have a certain minimum value for their kinetic energy, known as the activation energy, Ea Factors affecting rate of reaction |
Unit 7: Equilibrium
| Subtopic | Subtopic Number | IB Points to Understand |
| Equilibrium | 7.1 (SL) | A state of equilibrium is when both the forward and reverse reactions occur simultaneously with products and reactants constantly being interconverted.
The law of chemical equilibrium states that at a given temperature the ratio of the concentration of products to the concentration of reactants is a constant, Kc The effects of changing experimental conditions on the equilibrium constant The ratio of concentration of product to reactants will not equal K. This ratio is called the reaction quotient Q and it helps determine the progress of the reaction as it moves toward equilibrium. |
Unit 8: Acids and Bases

| Subtopic | Subtopic Number | IB Points to Understand |
| Theories of acids and bases | 8.1 (SL) | An acid is any hydrogen containing substance that is capable of donating a proton
A base is a molecule or ion able to accept a hydrogen ion from an acid Arrhenius theory: acids and bases states that “an acid generates H+ ions in a solution whereas a base produces an OH- ion in its solution” The combination of an acid and base is well known as a neutralization reaction involving the combination of the hydrogen ion and the hydroxide ion Bronsted-Lowry: the theory defines “an acid as a proton donor and a base as a proton acceptor” Lewis theory: The Lewis definition of acids and bases describes “acids as electron-pair acceptors and bases An amphiprotic substance is one which can act as both a proton donor and a proton acceptor |
| Properties of acids and bases | 8.2 (SL) | Physical properties of acids and bases
Neutralization reactions are used to calculate the exact concentration of an acid or an alkali when the other concentration is known. The solution of known concentration is known as the standard solution. The equivalence point is reached when the acid and the base exactly neutralize each other. A good indicator is one that gives a distinct or sharp color change at the equivalence point. |
| The pH scale | 8.3 (SL) | The pH scale is a simple and effective way of representing the concentration of hydrogen ions, [H+] in a solution
Calculating the pH A way to measure pH is with universal indicator paper, pH meter or solution Ionization of water The relationship between H+ and OH– is inverse |
| Strong and weak acids and bases | 8.4 (SL) | If the acid dissociates fully, it will exist entirely as ions in solution. It is said to be a strong acid
If the acid dissociates only partially, it produces an equilibrium mixture in which the undissociated form dominates. It is said to be a weak acid Distinguishing between strong and weak acids and bases using electrical conductivity, rate of reaction and their pH |
| Acid deposition | 8.5 (SL) | Acid deposition is the process by which acid-forming pollutants are deposited on the Earth’s surface
Acid rain refers to solutions with a pH below 5.6, and which therefore contain additional acids. The main contributors to acid rain are the oxides of sulfur and nitrogen Effects of acid deposition on building materials, plant life, water and human health Pre and Post-combustion technologies |
Unit 9: Redox Processes

| Subtopic | Subtopic Number | IB Points to Understand |
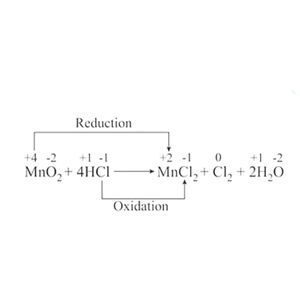
| Oxidation and Reduction | 9.1 (SL) | Oxidation describes a process in which the oxidation state increases and reduction describes a process in which the oxidation state decreases.
Oxidation and reduction will always occur together and reactions of this type are known as redox reactions. Oxidation state is the apparent charge of an atom in a free element, a molecule, or an ion. Half-equations can be very useful in balancing complex redox reactions. Each half-equation represents the separate oxidation and reduction processes. The reactant that accepts electrons is called the oxidizing agent as it brings about oxidation of the other reactant. In the process it becomes reduced. Likewise the reactant that supplies the electrons is known as the reducing agent, because it brings about reduction and itself becomes oxidized. |
| Electrochemical cells | 9.2 (SL) | Voltaic (or galvanic) cells – these convert chemical energy to electrical energy. Voltaic cells convert energy from spontaneous, exothermic chemical processes to electrical energy.
Electrolytic cells – these convert electrical energy to chemical energy, bringing about a non- spontaneous process. An electrode is a conductor of electricity used to make contact with a non-metallic part of a circuit, such as the solution in a cell The electrode where oxidation occurs is called the anode. The electrode where reduction occurs is called the cathode |
Unit 10: Organic Chemistry

| Subtopic | Subtopic Number | IB Points to Understand |
| Fundamentals of Organic Chemistry | 10.1 (SL) | Organic chemistry is the field of chemistry that studies carbon-based compounds
A homologous series is a series of compounds that can be grouped together based on similarities in their structure and reactions The alkenes and alkynes are two more hydrocarbon homologous series that contain carbon– carbon double and triple bonds, respectively. Members of a homologous series can be represented by the same general formula The empirical formula represents the simplest ratio of atoms present in a molecule. The molecular formula describes the actual number of atoms present in the molecule Other representations: Full structural formula, condensed structural formula and skeletal formula There are two possible arrangements for atoms that correspond to different molecules with different properties. Molecules, having the same molecular formula but different arrangements of the atoms, are known as structural isomers Primary, Secondary and Tertiary compounds Arenes are a class of compounds that are derived from benzene, C6H6. They form a special branch of organic compounds known as the aromatics |
| Functional Group Chemistry | 10.2 (SL) | Alkanes are the simplest hydrocarbons.
Common reactions studied in organic chemistry include substitution, addition, and elimination. Substitution is the replacement of individual atoms with other single atoms or with a small group of atoms. In an addition reaction, two molecules are added together to produce a single molecule, while elimination is the removal of two substituents from the molecule. Free-radical refers to a species that is formed when a molecule undergoes homolytic fission: the two electrons of a covalent bond are split evenly between two atoms resulting in two free-radicals that each have a single electron Heterolytic fission of a bond creates a cation and an anion, as the electrons involved in the bond are unevenly split between the two atoms Initiation, Propagation and Termination Alkenes are unsaturated hydrocarbons that contain at least one carbon–carbon double bond Test for unsaturation Polymerization of alkenes: Addition polymerization is the reaction of many small monomers that contain a carbon– carbon double bond, linking together to form a polymer. The oxidation of a primary alcohol is a two-stage process that first produces an aldehyde followed by a carboxylic acid. The oxidation of a secondary alcohol such as propan-2-ol results in the formation of a ketone Esterification is a reversible reaction that occurs when a carboxylic acid and an alcohol are heated in the presence of a catalyst The electron-deficient carbon is attacked by electron-rich species known as nucleophiles. An electrophile is an electron-poor species capable of accepting an electron pair. It acts as a Lewis acid. |
Unit 11: Measurement and data processing and analysis

| Subtopic | Subtopic Number | IB Points to Understand |
| Uncertainties and errors in measurement and results | 11.1 (SL) | Significant figures refer to the number of digits reflecting the precision of a given measurement. |
| Graphical techniques | 11.2 (SL) | Graphical techniques are an effective means of communicating the effect of an independent variable on a dependent variable
The slope of gradient of the line. Intercept is found using extrapolation or using the equation of a line Best-fit line |
| Spectroscopic identification of organic compounds | 11.3 (SL) | The degree of unsaturation or index of hydrogen deficiency (IHD) can be used to determine from a molecular formula the number of rings or multiple bonds in a molecule.
The degree of unsaturation is used to calculate the number of rings and π bonds present in a structure Electromagnetic Spectrum Infrared spectroscopy: using this type of spectroscopy, various functional groups can be identified in a molecule. The vibrational transitions correspond to definite energy levels. Proton nuclear magnetic resonance spectroscopy Mass spectrometry |
Unit 12: Atomic Structure
| Subtopic | Subtopic Number | IB Points to Understand |
| Electrons in atoms | 12.1 (HL) | First and second ionization energy
Periodic trends in ionization energies |
Unit 13: Periodicity
| Subtopic | Subtopic Number | IB Points to Understand |
| First-row d-block elements | 13.1 (HL) | A transition element is an element that has an atom with an incomplete d-sublevel or that gives rise to cations with an incomplete d-sublevel.
Characteristics of transition elements Transition metals are often found with different oxidation states Compounds that contain transition elements and in which the central metal ion is bonded, via coordinate bonding, to a group of molecules or ions (termed the ligands) are termed transition metal complexes Ligands classification: Monodentate and Polydentate Transition metals work as catalysts in chemical reactions Paramagnetic and Diamagnetic materials |
| Coloured complexes | 13.2 (HL) | The visible spectrum ranges from 400 nm to about 700 nm
Transition metals appear coloured because they absorb visible light Transition metals absorb light because the d orbitals split into two sub-levels |
Unit 14: Chemical Bonding and Structure

| Subtopic | Subtopic Number | IB Points to Understand |
| Covalent bonding and electron domain and molecular geometries | 14.1 (HL) | A single covalent bond consists of two electrons shared between two atoms A and B.
A double covalent bond consists of four electrons, two pairs, shared between two atoms A and B A triple covalent bond consists of six electrons, or three pairs, shared between two atoms A and B. Catalytic destruction of ozone |
| Hybridization | 14.2 (HL) | A hybrid orbital results from the mixing of different types of atomic orbitals on the same atom
When carbon forms four single bonds, it undergoes sp3 hybridization, producing four equal orbitals. When carbon forms a double bond, it undergoes sp2 hybridization, producing three equal orbitals. When carbon forms a triple bond, it undergoes sp hybridization, producing two equal orbitals. |

Unit 15: Energetics and Thermochemistry

| Subtopic | Subtopic Number | IB Points to Understand |
| Energy cycles | 15.1 (HL) | The first ionization energy is the minimum energy required to remove one mole of electrons from one mole of gaseous atoms.
The first electron affinity is the enthalpy change when one mole of gaseous electrons is added to one mole of gaseous atoms. Born–Haber cycle and enthalpy of formation The lattice enthalpy is defined as the standard enthalpy change that occurs on the formation of 1 mol of gaseous ions from the solid lattice The enthalpy of atomization ΔHat is the standard enthalpy change that occurs on the formation of 1 mol of separate gaseous atoms of an element The ionization energy, ΔHIE, is the standard enthalpy change that occurs on the removal of 1 mol of electrons from 1 mol of atoms or positively charged ions in the gaseous phase. Enthalpy change if solution and hydration |
| Entropy and spontaneity | 15.2 (HL) | The combination of enthalpy, entropy, and temperature of system can be used to define a new state function called Gibbs free energy
The Gibbs free energy change of formation, ΔGf, represents the free energy change when 1 mol of a compound is formed from its elements under standard conditions of 298 K and a pressure of 100 kPa |
Unit 16: Chemical Kinetics

| Subtopic | Subtopic Number | IB Points to Understand |
| Rate expression and reaction mechanism | 16.1 (HL) | A catalyst is a substance that increases the rate of a chemical reaction, but is not consumed in the reaction itself. A catalyst provides an alternative pathway for the reaction and lowers the activation energy, Ea
Reactions may occur by more than one step and the slow step determines the rate of the reaction. The slow step is termed the rate-determining step (RDS Zero to third order reaction |
| Activation energy | 16.2 (HL) | The activation energy is the minimum amount of energy required for reaction to take place.
Arrhenius equation |
Unit 17: Equilibrium

| Subtopic | Subtopic Number | IB Points to Understand |
| The equilibrium law | 17.1 (HL) | Calculating equilibrium constant using the ICE Method
The Gibbs free energy change ∆G for a given reaction is an indication of whether the forward or reverse reaction is favored. |
Unit 18: Acids and Bases
| Subtopic | Subtopic Number | IB Points to Understand |
| Lewis acids and bases | 18.1 (HL) | A Lewis acid is a lone pair acceptor.
A Lewis base is a lone pair donor A nucleophile (‘likes nucleus’) is an electron-rich species that donates a lone pair to form a new covalent bond in a reaction An electrophile (‘likes electrons’) is an electron-deficient species that accepts a lone pair from another reactant to form a new covalent bond |
| Calculations involving acids and bases | 18.2 (HL) | Calculating acid dissociation constant Ka and the base dissociation constant Kb
The relationship between the acid dissociation constant for a weak acid and the base dissociation constant of its conjugate base can be useful in calculation The stronger the acid, the larger the Ka The weaker the acid, the larger the pKa |
| pH curves | 18.3 (HL) | A buffer is a solution that resists a change in pH upon the addition of small amounts of a strong base or strong acid, or upon the dilution of the buffer through the addition of water
The change in pH does not show a linear relationship with the volume of base added, partly due to the logarithmic nature of the pH scale
An indicator is typically a weak acid or a weak base that displays a different color in acidic or alkaline environments Different indicators must be used for different titrations, depending on the pH at the equivalence point. |
Unit 19: Redox Processes
| Subtopic | Subtopic Number | IB Points to Understand |
| Electrochemical cells | 19.1 (HL) | Electroplating – It is a process of deposition of a thin layer of a metal over another using an electrolytic process.
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use for reference on all half-cell potential reactions. A voltaic cell generates an electromotive force (EMF) resulting in the movement of electrons from the anode to the cathode via the external circuit. |
Unit 20: Organic Chemistry
| Subtopic | Subtopic Number | IB Points to Understand |
| Types of organic reactions | 20.1 (HL) | SN2 reactions and primary halogenoalkanes: Nucleophilic substitution in primary halogenoalkanes proceed in one step. The rate- determining step involves both the halogenoalkane and the nucleophile.
Drawing mechanisms for SN2 reactions SN1 reactions and tertiary halogenoalkanes: Tertiary halogenoalkanes undergo nucleophilic substitution reactions that involve two steps. The rate-determining step involves only the halogenoalkane. Drawing mechanisms for SN1 reactions The rate of a nucleophilic substitution reaction depends on three main factors: Identity of the halogen, Classes of the halogenoalkane and the Choice of Solvent The major products of the electrophilic addition of hydrogen halides to unsymmetrical alkenes can be predicted using Markovnikov’s rule. Electrophilic addition of halogens to alkanes and interhalogens to alkenes Interhalogens are compounds in which two or more halogens are combined in a molecule. Electrophilic substitution reactions and its drawing mechanism Carboxylic acids are reduced to aldehydes and eventually to primary alcohols while ketones are reduced to secondary alcohols, in reactions that are the reverse of the oxidation of alcohols. |
| Synthetic routes | 20.2 (HL) | Organic synthesis takes a starting material and converts it via a series of reactions into the desired product.
Retrosynthesis |
| Stereoisomerism | 20.3 (HL) | Stereoisomers have an identical molecular formula and bond multiplicity but show different spatial arrangements of the atoms.
Stereoisomers can be subdivided into two major classes, conformational isomers and configurational isomers. Conformational isomers therefore differ from one another in the arrangement of atoms around a single bond. Configurational isomers can be interconverted only by the breaking of bonds or through the rearrangement of the stereocenters. Cis–trans isomers are determined by the positions of substituents relative to a reference plane. Optical isomerism is a type of configurational isomerism determined by the presence of chiral carbon atoms. Plane polarized light Diastereomers and Enantiomers |
Unit 21: Measurement and Analysis
| Subtopic | Subtopic Number | IB Points to Understand |
| Spectroscopic Identification of organic compounds | 21.1 (HL) | A high-resolution 1HNMR spectrum can show further splitting of some absorptions.
Splitting patterns result from spin–spin coupling. Single X-ray Crystallography is a scientific method used to determine the arrangement of atoms of a crystalline solid in three dimensional space. |
OPTION A: Materials

| Subtopic | Subtopic Number | IB Points to Understand |
| Materials science introduction | A.1 (SL) | Metallic substances exhibit metallic bonding. This makes them strong, malleable, and good conductors of heat and electricity.
Ceramics are traditionally inorganic non-metallic solids formed between metals and non- metals. They have a crystalline structure Polymers form a third classification of materials based on bonding. Plastics are covalently bonded long chain molecules Composites are mixtures composed of two distinct phases: a reinforcing phase embedded in a matrix. Bond triangle diagram |
| Metals and inductively coupled plasma (ICP) spectroscopy | A.2 (SL) | Reduction of iron ore in the blast furnace
Reduction by a more reactive metal Alloys are homogeneous mixtures of metals with other metals or non-metals. Paramagnetic materials are attracted to a magnetic field whereas diamagnetic materials create a magnetic field opposed to the applied field; and are therefore weakly repelled by an external magnetic field. In a ferromagnetic material the electron alignment induced by the magnetic eld can be retained, making a permanent magnet. Spectroscopic methods: atomic emission spectroscopy, optical emission spectroscopy, mass spectrometry |
| Catalysts | A.3 (SL) | A homogeneous catalyst is in the same phase as the reactants, takes the part of a reactant, and is reformed as a product at the end of the reaction.
A heterogeneous catalyst is in a different phase than that of the reactants. The mechanism by which the activation energy is lowered varies between homogeneous and heterogeneous catalysts. Nanocatalysts and Transition metal catalysts Zeolites: They are microporous substances made of alumina silicate which has a cage-like structure providing a large surface area |
| Liquid crystals | A.4 (SL) | Liquid crystals are a state of matter intermediate between crystalline and liquid.
Thermotropic liquid-crystal materials are pure substances that show liquid-crystal behavior over a temperature range between the solid and liquid states. Lyotropic liquid crystals are solutions that show the liquid-crystal state at certain concentrations. The nematic liquid-crystal phase is characterized by rod-shaped molecules that are randomly distributed but on average align in the same direction. Forming LCD displays |
| Polymers | A.5 (SL) | A polymer is a molecule i.e. formed by joining many small molecules called monomers.
Polymers can be classified as thermoplastics and thermosets based on their behavior when heated. Elastomers are flexible polymers that return to their original shape after being deformed. Polyvinyl chloride (PVC), is a synthetic resin made from the polymerization of vinyl chloride (chloroethene). Polystyrene (polyphenylethene, (C8H8)n) is a thermoplastic polymer made from the monomer styrene. Isotactic, Atactic, and Syndiotactic addition polymers Identifying monomers Atom economy is the measure of the amount of starting materials that end up as useful/desired products. |
| Nanotechnology | A.6 (SL) | Nanotechnology deals with the manipulation and control of atoms, molecules, and objects with dimensions of less than 100 nm
Methods of producing nanotubes include arc discharge, chemical vapor deposition (CVD), and high pressure carbon monoxide disproportionation (HiPCO) Arc Discharge using carbon electrodes and metal electrodes Chemical vapor deposition |
| Environmental impact: Plastics | A.7 (SL) | Green chemistry, also known as sustainable chemistry, is the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances.
The name “dioxins” refers to a class of environmental pollutants that are POPs. Certain dioxin-like polychlorinated biphenyls (PCBs) with similar toxic properties are sometimes included in the term “dioxins” Recycling of plastics |
| Superconducting metals and X-ray crystallography | A.8 (HL) | Superconductors are materials that offer no resistance to electric currents below a critical temperature.
The Meissner effect is the ability of a superconductor to create a mirror image magnetic field of an external field, thus expelling it. Resistance in metallic conductors is caused by collisions between electrons and positive ions of the lattice. The Bardeen–Cooper–Schrieffer (BCS) theory explains that below the critical temperature electrons in superconductors form Cooper pairs which move freely through the superconductor. Type 1 superconductors have sharp transitions to superconductivity whereas Type 2 superconductors have more gradual transitions. X-ray diffraction can be used to analyze structures of metallic and ionic compounds. Crystal lattices contain simple repeating unit cells. The number of nearest neighbors of an atom/ion is its coordination number. |
| Condensation polymers | A.9 (HL) | Condensation polymers are polymers formed through a condensation reaction where molecules join together, losing small molecules as byproducts such as water or methanol.
In condensation polymerization, many monomers are joined by condensation reactions to form the polymer. The esterification reaction: Acyl chlorides react with amines rather than alcohols in a condensation reaction that forms an amide. Phenol-methanol plastics, polyurethanes Polymer properties |
| Environmental impact: Heavy metals | A.10 (HL) | “Heavy metals” is a term that refers to toxic metals which have cumulative effects on health.
Chelating agents are used to remove heavy metals such as lead, arsenic, and mercury from the body. Absorption of heavy metals |
Option B: Biochemistry
| Subtopic | Subtopic Number | IB Points to Understand |
| Introduction to Biochemistry | B.1 (SL) | Biochemistry studies chemical processes in living cells at the molecular level.
Anabolic reactions produce large organic molecules from simpler organic or inorganic substances while catabolic reactions, complex molecules are broken down into smaller fragments The primary chemical element in all biologically important molecules is carbon Photosynthesis, respiration and the atmosphere |
| Proteins and enzymes | B.2 (SL) | Proteins are the most diverse and abundant class of biopolymers, responsible for over 50% of the dry mass of cells
The structural units of proteins are joined together by amide linkages (also known as peptide Shorter polymers composed of less than 20 residues of 2-amino acids are called peptides The first amino acid in a peptide has a free NH2 group, described as N-terminal, while the last amino acid has an unreacted COOH group (C-terminal) Properties of peptides Long chains of amino acid residues in proteins tend to adopt certain highly ordered conformations, such as α-helix and β-pleated sheet Enzymes |
| Lipids | B.3 (SL) | Lipids are a broad group of naturally occurring substances that are largely non-polar and therefore insoluble in water
Fatty acids are long-chain unbranched carboxylic acids. Saturated fatty acids contain only single carbon–carbon bonds and have the general formula Unsaturated fatty acids with one or more CH=CH groups in their molecules are described as Calculating energy content Steroids are a class of lipids with a characteristic arrangement of three six-membered and one five-membered hydrocarbon rings fused together in a specific order Cholestrol’s solubility in blood plasma is extremely low. In the human body cholesterol is transported as a lipoprotein. They can be classified as low-density lipoproteins (LDL) or high-density lipoproteins (HDL) Most steroids are hormones |
| Carbohydrates | B.4 (SL) | Carbohydrates are a family of oxygen-rich biomolecules that play a central role in the metabolic reactions of energy transfer
Monosaccharides with five and six carbon atoms in their molecules are known as pentoses and hexoses, respectively Monosaccharides with a carbonyl group at the second carbon atom are known as ketoses Glucose is an important intermediate in various metabolic processes Glycogen is structurally similar to amylopectin but is more densely branched and contains up to a million glucose residues. |
| Vitamins | B.5 (SL) | Vitamins are organic micronutrients that cannot be synthesized by the organism in sufficient amounts and must either be obtained from suitable foods or taken as food supplements
Preventing deficiencies Vitamin A: Retinoids and Carotenoids – relatively stable to heat Vitamin C: Ascorbic acid – water-soluble vitamin C is unstable at high temperatures Vitamin D: Cholecalciferol – relatively stable to heat |
| Biochemistry and the environment | B.6 (SL) | Biochemistry studies the chemical changes associated with living organisms and their interactions with the environment
Xenobiotics are generally toxic to various life forms and are more resistant to biodegradation than naturally occurring organic molecules Host–guest complexes mimic the structures of enzyme–substrate complexes, where the host and the guest are held together by non-covalent interactions Non-biodegradable materials are the most abundant and persistent environmental pollutants produced by humans |
| Proteins and Enzymes | B.7 (HL) | Depending on the solution pH, the carboxyl and amino groups in these amphoteric compounds can be ionized to various extents producing ionic species with different charges.
Amino acids can act as acid–base buffers only within certain pH ranges Proteins as biological buffers The detection of proteins and the determination of their concentrations in solutions is known as protein assay |
| Nucleic acids | B.8 (HL) | Heredity and the storage of biological information in the nucleus of the cell
Nucleic acids are condensation polymers of nucleotides, which in turn are the products of condensation of a nitrogenous base, a pentose sugar, and phosphoric acid. DNA molecules consist of two polynucleotide strands The double-helix shape of the DNA molecule stabilized by hydrogen bonds between complementary nitrogenous bases is known as its secondary structure. DNA replication is facilitated by several families of enzymes and includes three steps: initiation, elongation, and termination Initiator proteins separate the two DNA strands and create short polynucleotide fragments (primers) paired with the separated strands by complementary nitrogenous bases. DNA polymerases add more nucleotides to the primers using the existing DNA strands as templates A mechanism similar to replication is used when an RNA molecule is created from a DNA template in a process called transcription Genetic engineering allows scientists to alter DNA sequences in the genes of living organisms, including the transfer of genetic material between different species. |
| Biological Pigments | B.9 (HL) | Most organic compounds are colorless because they do not absorb electromagnetic radiation in the visible range of the spectrum.
Carotenes Porphyrins are complexes of metal ions with large cyclic ligands. Porphin contains four nitrogen atoms in a highly conjugated aromatic heterocycle. Cytochromes Chlorophyll Photosynthesis is a complex process that involves many pigments and proteins collectively known as photosystems |
| Stereochemistry in biomolecules | B.10 (HL) | Stereoisomers: molecules that have the same sequence of atoms and chemical bonds but different arrangements of atom
Stereoisomers that cannot be transformed into one another without breaking a chemical bond are called configurational isomers and include two classes: cis-/transisomers and optical isomers. The hydrocarbon chains in unsaturated fatty cannot adopt linear conformations Trans-fats: If the hydrogenation is incomplete, the final product will contain trans-unsaturated triglycerides Retinal, is a long-chain aldehyde involved in vision chemistry The aldehyde group of cis-retinal can reversibly bind to a lysine residue of the protein opsin, producing a light-sensitive pigment rhodopsin |
Option C: Energy

| Subtopic | Subtopic Number | IB Points to Understand |
| Energy sources | C.1 (SL) | Quality and efficiency of energy sources
Some renewable energy resources include solar energy, wind energy, biomass, water, geothermal energy, and fuel cells |
| Fossil fuels | C.2 (SL) | Fossil fuels contain saturated alkanes
Cracking converts longer-chain hydrocarbons into more useful shorter chain alkenes and alkanes A measure of the fuel’s ability to resist auto-ignition is its octane rating. Catalytic reforming is used to convert low-octane numbered alkanes into higher-octane numbered isomers Carbon capture and storage (CCS) involves capturing carbon dioxide from large industrial processes, compressing it, and transporting it to be injected deep into rock formations at selected safe sites The carbon footprint of a reaction is a measure of the net quantity of carbon dioxide produced by the process. |
| Nuclear fusion and fission | C.3 and C.7 (SL) | Hydrogen fusion
By fusing lighter elements to form larger ones the binding energy increases and the mass defect is converted to energy. The heavier transuranium elements (those with atomic number greater than 92) can undergo nuclear fission to form two lighter nuclei. The amount of material needed for the reaction to remain sustainable is the critical mass. The conversion of one element to another by capture or emission of a particle is referred to as transmutation. The half-life (t1/2) refers to the time it takes for one half of the number of atoms in a sample to decay. The mass defect is mass of the nucleus – the sum of the masses of its nucleons The nuclear binding energy (ΔE) is the energy required to separate a nucleus into protons and neutrons. |
| Solar energy | C.4 (SL) | Visible light can be absorbed by molecules that have a conjugated structure with an extended system of alternating single and multiple bonds.
Biofuels such as ethanol are obtained from corn sugar or glucose by fermentation Advantages and disadvantages of biodiesel |
| Environmental impact: Global warming | C.5 (SL) | These radiation waves interact with greenhouse gasses, which capture this energy so that it remains trapped in the Earth’s atmosphere.
This natural effect of the atmosphere is ‘greenhouse effect’. Natural sources of greenhouse gasses Greenhouse gas emission from human activities Of all the carbon dioxide gas released to the atmosphere by human activity, approximately half has remained in the atmosphere. The rest is removed to carbon sinks. Carbon capture and storage (CCS) is the process of capturing waste carbon dioxide from where it is produced, transporting it to a storage site, and storing it where it will not enter the atmosphere Agriculture and deforestation – Methane, CH4 and nitrous oxide, N2O are the main greenhouse gasses produced in agriculture. Effects of climate change include melting permafrost, less radiation reaching the Earth’s surface, devastating storms, temperatures becoming more extreme (both hotter and colder), and record levels of rainfall and droughts. |
| Electrochemistry, rechargeable batteries, and fuel cells | C.6 (HL) | A battery is a series of portable electrochemical cells.
In a primary electrochemical cell the materials are consumed and the reaction is not reversible. In a secondary cell or rechargeable battery the chemical reactions that generate electricity can be reversed by applying an electric current to them. Lithium-ion rechargeable batteries use lithium atoms absorbed into a lattice of graphite electrodes The further apart the standard electrode potentials of the oxidizing and reducing materials, the more voltage per cell is available A fuel cell is an electrochemical device that converts the chemical potential energy in a fuel into electrical energy PEM fuel cell and its key components Alkali and microbial fuel cells |
| Photovoltaic and dye sensitized solar cells (DSSC) | C.8 (HL) | Conjugation is the interaction of alternating double bonds, for example in organic molecules, to produce a delocalized array of pi electrons over all the atoms
The photovoltaic cell absorbs photons in a semiconducting material, which causes some valence electrons to be removed, resulting in some ionization in the cell. The conductivity of the semiconductor can be increased by “doping” it with small impurities to create an n-type semiconductor, or p-type semiconductor. Dye sensitized solar cells |
Option D: Medicinal Chemistry
| Subtopic | Subtopic Number | IB Points to Understand |
| Pharmaceutical products and drug action | D.1 (SL) | Medicinal chemistry is a cross-disciplinary science that links together organic chemistry, pharmacology, biochemistry, biology, and medicine
Drug: a chemical that affects how the body works. Medicine: a substance that improves health. Side-effects When a person is given repeated doses of a drug, tolerance can develop. Addiction is when a patient becomes dependent on the drug in order to feel normal, and suffers from withdrawal symptoms if the drug is not taken. The dosing regime for a drug refers to the specific quantity of drug to be taken at one time, and the frequency of administration. Drug action and development of new drugs |
| Aspirin and Penicillin | D.2 (SL) | The chemically modified salicylic acid, known as acetylsalicylic acid or aspirin
Aspirin and salicylic acid belong to the class of mild analgesics, also known as non-narcotic analgesics and non-steroidal anti-inflammatory drugs (NSAIDs). Because aspirin is almost insoluble in water. The solubility and bioavailability of pharmaceutical drugs can be increased by converting them into ionic salts. Development of penicillin into a drug Certain bacteria mutated and developed varying degrees of antibiotic resistance due to increased production of the enzyme penicillinase. This enzyme was able to deactivate benzylpenicillin and prevent it from binding to transpeptidase. |
| Opiates | D.3 (SL) | The primary bioactive ingredient of opium, morphine, is a natural analgesic that belongs to the group of alkaloids – naturally occurring chemical compounds containing basic nitrogen atoms.
Morphine and its derivatives (opiates) are strong analgesics The physiological activity of opiates strongly depends on their ability to cross the blood-brain barrier. Codeine readily crosses this barrier but does not bind to the opioid receptor because of the steric effect of the ester group. The polarity of morphine can be reduced by chemical modification of one or both hydroxyl groups in its molecule. Diamorphine |
| pH regulation of the stomach | D.4 (SL) | The process of digestion involves a series of catabolic reactions that transform food nutrients into small molecules. Food is mixed with a digestive fluid called gastric juice
Antacids can quickly increase the pH of gastric juice by reacting with hydrochloric acid. The acidity of gastric juice can be controlled at the cellular level by targeting the biochemical mechanisms of acid production. Omeprazole and esomeprazole: They have the same molecular formula and differ in their stereoisomeric structure. Acid-base buffers & Hydrogen Carbonate and carbonate buffers |
| Antiviral medications | D.5 (SL) | Antibiotics are completely ineffective against viruses
Most viruses are nucleoproteins containing a nucleic acid (RNA or DNA) surrounded by a protein coat. This coat, known as a capsid, consists of multiple protein units arranged in helical or polyhedral structures. Since viruses are not alive, they cannot be “killed” by drugs; instead antivirals interfere with different stages of the virus replication cycle The human immunodeficiency virus (HIV), is responsible for acquired immunodeficiency syndrome (AIDS), which is characterized by progressive failure of the immune system and the development of life-threatening opportunistic infections and cancers. |
| Environmental impact of some medications | D.6 (SL) | Pharmacologically active compounds (PACs) used in medicine and biochemical studies have not been treated as potentially toxic and have been routinely released to the environment.
Many medical procedures involve the use of radionuclides – unstable isotopes of certain elements that undergo spontaneous radioactive decay. The waste containing such radionuclides is known as low-level waste (LLW) High-level waste (HLW) is produced in nuclear reactors and contains a mixture of nuclear fission products with unused nuclear fuel. Organic solvents used in the pharmaceutical industry constitute a significant proportion of chemical waste. Green Chemistry |
| Taxol: a chiral auxiliary case study | D.7 (HL) | The discovery and development of the anticancer drug paclitaxel (Taxol®)
Automatic extractors can process and analyse hundreds of fractions, discarding empty extracts and combining similar fractions for further separation. Semi synthetic production Small amounts of Taxol are still isolated from Pacific yew using advanced techniques such as extraction with supercritical carbon dioxide. |
| Nuclear medicine | D.8 (HL) | Nuclear medicine uses radioactive materials in the diagnosis and treatment of diseases.
Unstable isotopes can be combined with biologically active compounds, producing radiopharmaceuticals – drugs that deliver radionuclides to specific tissues or cellular The primary use of radiotherapy is the treatment of cancer. The three most common types of radiation (alpha particles, beta particles, and gamma rays) Targeted alpha therapy (TAT), Boron neutron capture therapy (BNCT), Proton beam therapy (PBT) Bragg’s peak effect allows the proton beam to be focused on the tumor with minimal radiation damage to healthy tissues. Magnetic resonance imaging (MRI) is a medical application of nuclear magnetic resonance. |
| Drug detection and analysis | D.9 (HL) | A variety of analytical techniques is used for the detection and analysis of pharmaceutical drugs.
Spectroscopic identification of drugs Many natural and synthetic products used in pharmaceutical chemistry have to be isolated from their mixtures with other compounds – Extraction and purification of organic products Fractional Distillation Drug detection in sports and forensic studies |
Frequently Asked Questions (FAQs)
Q1: What topics are covered in the IB Chemistry syllabus?
A: The IB Chemistry syllabus covers a wide range of topics, including atomic structure, bonding, energetics, kinetics, equilibrium, acids and bases, oxidation and reduction, organic chemistry, and analytical chemistry.
Q2: How is the IB Chemistry course assessed?
A: The IB Chemistry course is assessed through a combination of internal and external assessments. Internal assessments include lab work, field work, and other practical activities, while external assessments include written exams and a scientific investigation.
Q3: What skills do students need to succeed in IB Chemistry?
A: Students in IB Chemistry need to have strong analytical, problem-solving, and experimental design skills. They should also be able to communicate scientific ideas clearly and accurately, and be comfortable with data analysis and statistical analysis.
Q4: How is the IB Chemistry course different from other high school chemistry courses?
A: The IB Chemistry course is designed to be more rigorous and in-depth than other high school chemistry courses. It emphasizes a conceptual understanding of chemical concepts, as well as practical skills such as experimental design, data analysis, and scientific communication.
Q5: What are the benefits of taking the IB Chemistry course?
A: Taking the IB Chemistry course can provide students with a strong foundation in chemistry that will prepare them for future studies in the field. It can also help students develop critical thinking and problem-solving skills that are valuable in a wide range of careers. Additionally, the IB Chemistry course is recognized by colleges and universities around the world, which can be beneficial for college admissions.