Atmospheric Systems and Societies Notes
Atmospheric Systems and Societies
6.1 Introduction to Atmosphere
6.1.1 Atmosphere as a Dynamic System
The Earth’s atmosphere is both influenced by the biosphere and influences the biosphere. The current atmosphere consists of nitrogen (78.1%), oxygen (20.9%), and carbon dioxide (0.4%). If the effects of the biosphere were removed, it is estimated that the atmospheric composition would be 1.9% nitrogen, 0% oxygen and 98% carbon dioxide. This illustrates the close relationship between the biosphere and the atmosphere.
The atmosphere continues to change as a result of natural and human-induced processes. The high levels of carbon dioxide in the atmosphere have resulted in higher temperatures since the dawn of industrialization. The following factors have contributed to the higher mean global temperatures every year:
- The onset of global industrialization and the subsequent production of pollution derived from fossil fuels
- Deforestation, particularly of rainforest
- Volcanic activity
- Sunspot activity.
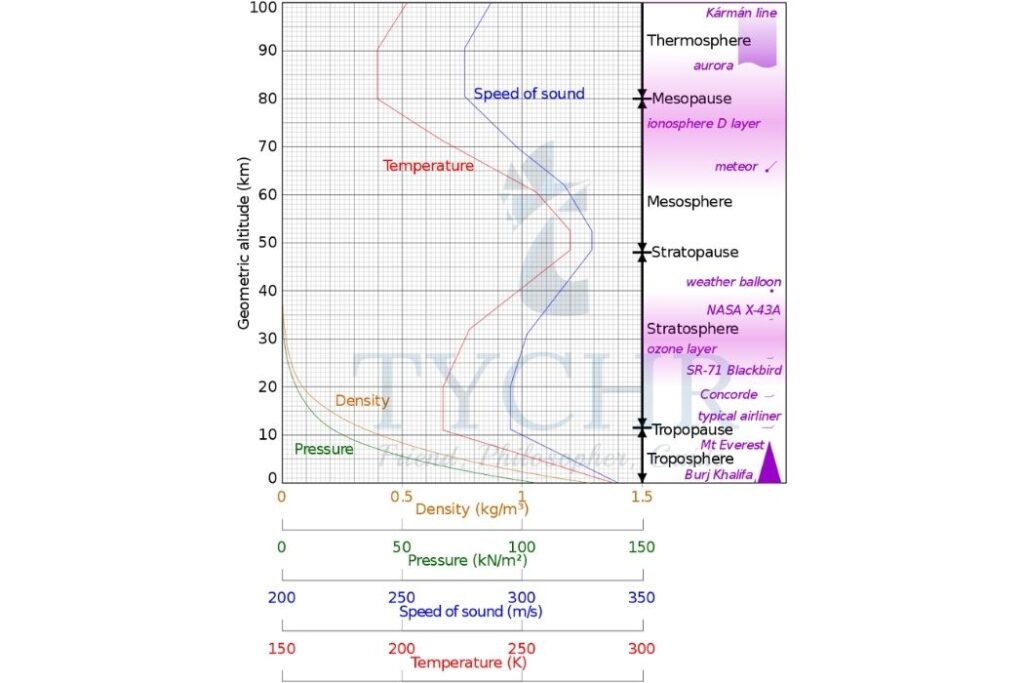
Variations in Composition with Altitude
The concentrations of gases have an important effect on changes in temperature through the atmosphere. At the tropopause, there is a reversal in the temperature gradient. This acts as the upper limit of weather systems. In the stratosphere, the increase in temperature is related to the presence of ozone. Temperatures then fall in the mesosphere but increase again in the thermosphere.
6.1.2 Earth’s Energy Budget
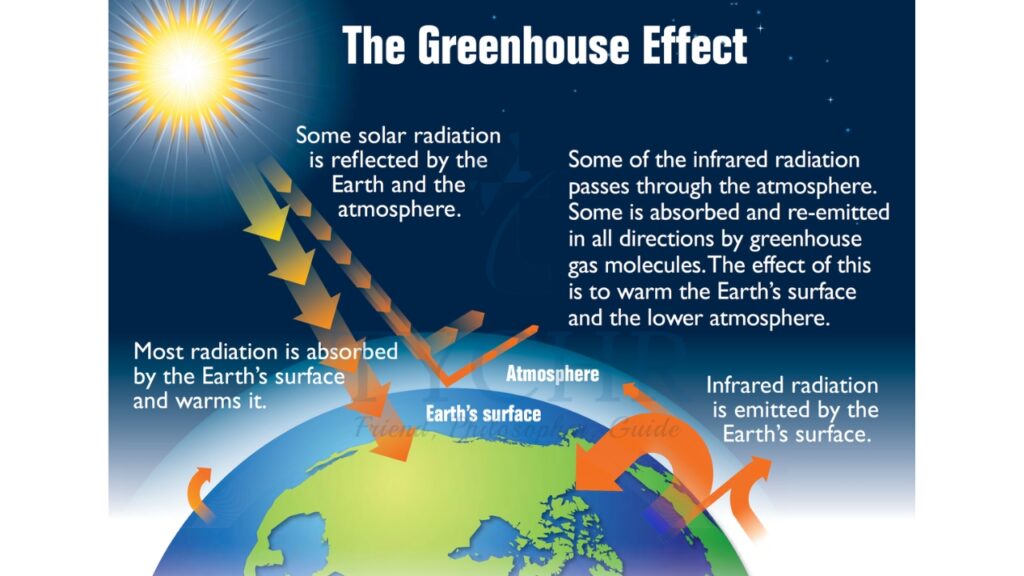
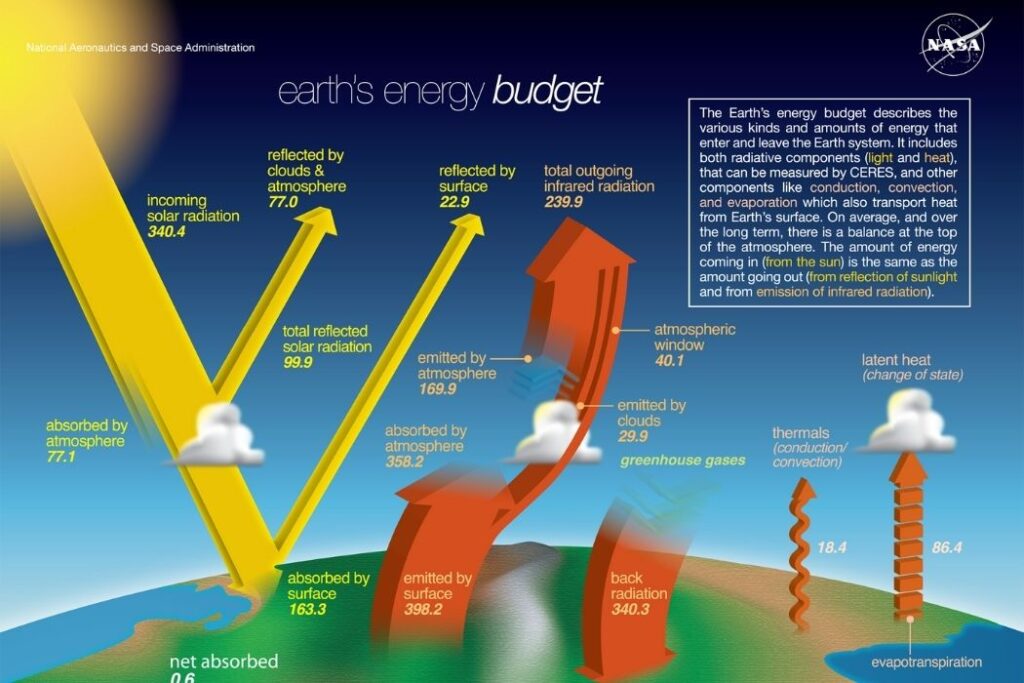
The Earth’s energy is mostly derived from the Sun. Insolation refers to incoming solar radiation. Solar energy (short-wave energy) is received by the Earth and its atmosphere and transformed in a number of processes as illustrated in the figure. For every 100 units of solar energy reaching the Earth’s atmosphere, 31% is reflected back to space, and 69% is absorbed by the Earth and the atmosphere.
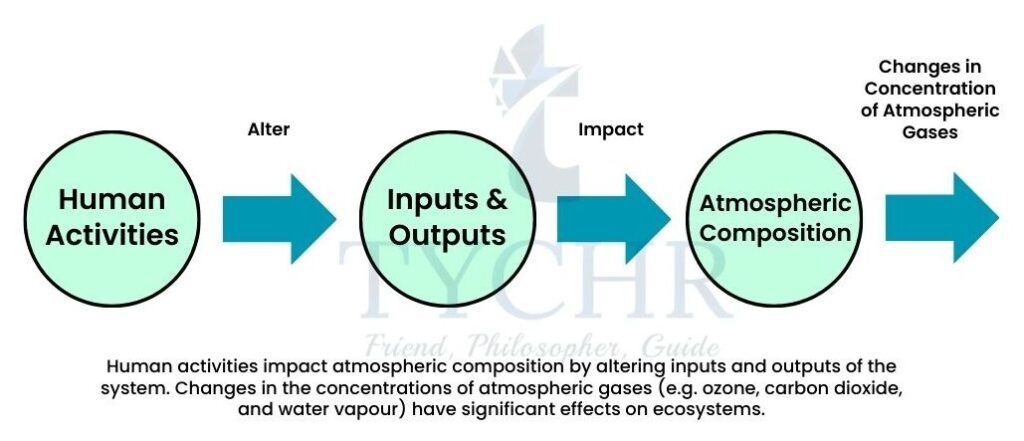
6.1.3 Human Activities and Atmospheric Composition
The main human activities releasing greenhouse gases are as follows:
- Burning of fossil fuels (that release carbon dioxide.
- Deforestation, which results in release of carbon dioxide due to breakdown in forest biomes.
- Increased intensive cattle farming has led to increased methane levels. This is because cattle ranching increases as the demand for beef increases.
- Fertilisers in agricultural systems has led to higher nitrous oxide (N2O) concentrations when the
fertilisers break down.
6.1.4. The Greenhouse Effect
- The atmosphere maintains the temperature of the Earth.
- Within the atmosphere, certain gases trap the radiation that heats the surface.
- Shortwave ultraviolet (UV) light from the Sun is reflected from the surface of the Earth as infrared (IR) light (which has a longer wavelength). The process is sometimes known as ‘radiation trapping’.
- This effect is caused mainly by water vapour and carbon dioxide. Other gases involved are methane (CH4), nitrous oxide (N2O), and ozone (O3).
- The gases create a ‘thermal blanket’ that maintains an average Earth temperature that can support life.
- Because these gases act in the same way that glass acts in a greenhouse, they are called greenhouse gases and the effect they have is called the greenhouse effect.
Let’s Revise
Troposphere – the lowest layer of the atmosphere extending from the ground’s surface to the tropopause (between 10 km and 15 km).
Stratosphere – a layer of the Earth’s atmosphere extending from the tropopause to about 50 km.
Albedo – the amount of incoming radiation that is reflected by the Earth’s surface and atmosphere.
6.2 Stratospheric Ozone
6.2.1 UV Radiation and Ozone
Ozone is essential for sustaining life. The highest concentration of ozone occurs in the upper part of the atmosphere, the stratosphere, where it is formed through the action of UV radiation on oxygen. The ozone layer shields the Earth from harmful radiation that would otherwise destroy most life on the planet.
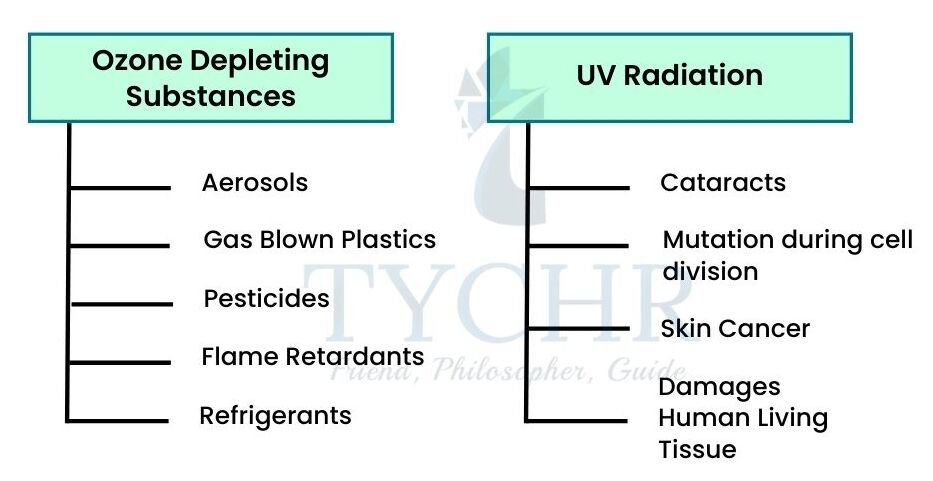
6.2.2 Ozone Depleting Substances Halocarbons
The chemicals that cause stratospheric ozone depletion include halocarbons. Halogens include chlorofluorocarbons (CFCs). These are found in many products, including aerosols and refrigerators. They can also be found in air, are sometimes found in pesticides and are also used for fire extinguishers and solvents.
Holes in the Ozone Layer
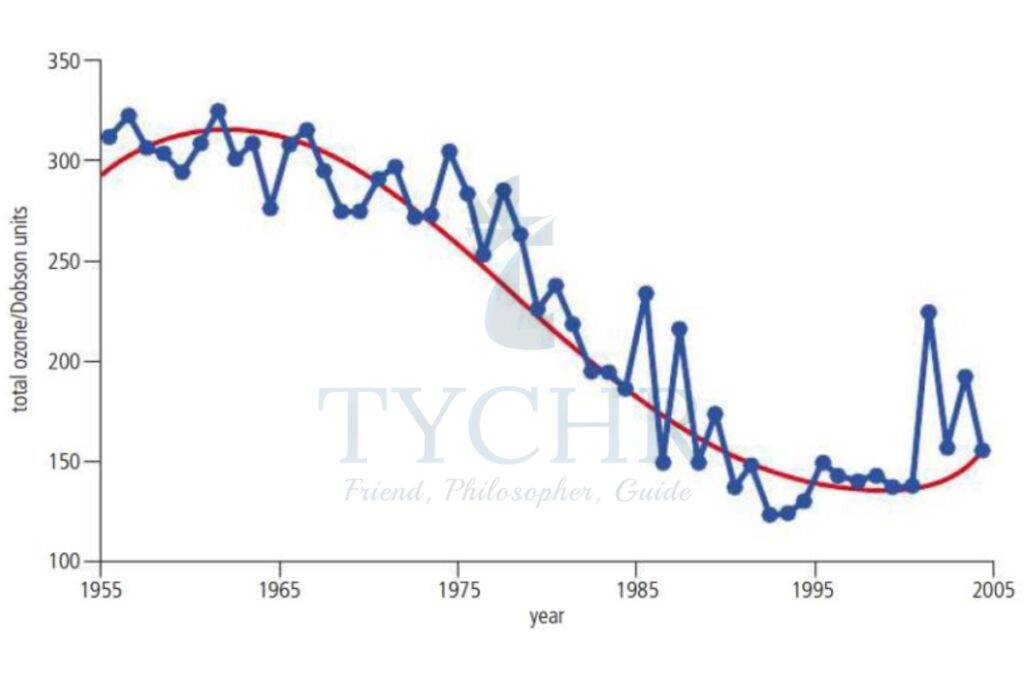
Pollutants increase the destruction of ozone. They change the equilibrium of the ozone production system. They cause “holes” in the ozone layer. The ozone hole is a thinning of the concentration of ozone in the stratosphere. The ozone “hole” allows more ultraviolet radiation to pass through the Earth’s atmosphere. This can be very damaging.
6.2.3 Effects of Ultraviolet Radiation on Human Health
- Increased ultraviolet radiation is damaging to ecosystems as it damages plant tissues and plankton.
- Effect on Aquatic Ecosystems- Ultraviolet radiation damages marine phytoplankton, which is one of the major primary producers of the biosphere. It causes reduced rates of photosynthesis. In aquatic ecosystems the organisms that live in the upper part of the water are most affected.
- UV radiation can also cause genetic mutations in DNA. There are also negative impacts on reproduction.
- Eye Damage and Skin Cancer- It causes cataracts. The effects of long-term exposure are irreversible and can cause blindness. It can also cause sunburn and eventually skin cancer. Research suggests annual loss of ozone is about 1% and there has been an increase in skin cancers of 4%.
6.2.4 Reducing Ozone-Depleting Substances
Recycling Refrigerants | Alternatives to Gas- Blown Plastics and Propellants | Phase out of Methyl Bromide |
|
|
|
6.2.5 National and International Organizations and the Reduction of ODSs
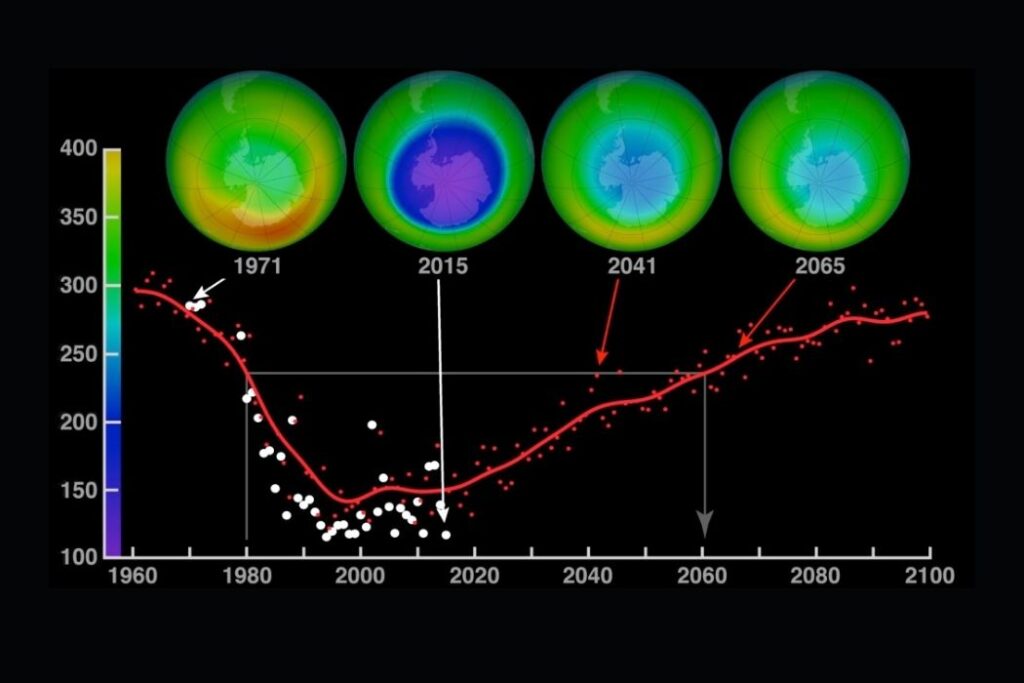
- The 1987 Montreal Protocol on Substances that Deplete the Ozone Layer is the most significant and successful international agreement relating to an environmental issue. Nearly 200 governments have signed up and implemented the agreed changes according to the Montreal Protocol.
- It is believed that ozone could recover by 2050 as a result of the Montreal Protocol.
- Subsequent revisions have reduced the phasing out timescale because of success—phase-out in Europe was achieved by 2000. Total global phase-out is expected by 2030.
- The Protocol incentivized countries to find alternatives. It raised public awareness of the use of CFCs.
- Technology has been transferred to LEDCs to allow them to replace ozone-depleting substances. The success of the protocol depends on national governments agreeing to its requirements.

6.3 Photochemical Smog
6.3.1 Source and Impact of Tropospheric Ozone
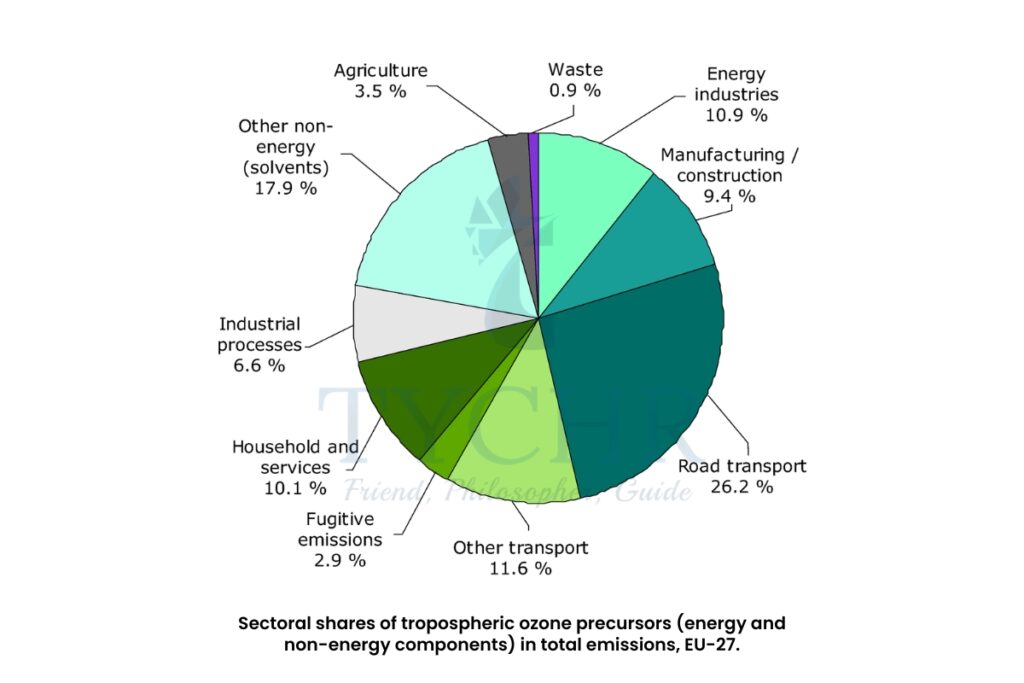
- Smog is formed as the result of pollution by volatile organic compounds (VOCs), carbon monoxide, carbon dioxide, black carbon or soot, unburned hydrocarbon, oxides of nitrogen (NOx), and oxides of sulphur.
- Primary pollutant- The main cause of photochemical smog (ground level/tropospheric ozone) is the volume of road transport concentrated in cities. Pollutants including hydrocarbons, carbon monoxide, carbon dioxide and nitrogen monoxide are released when fossil fuels are burned.
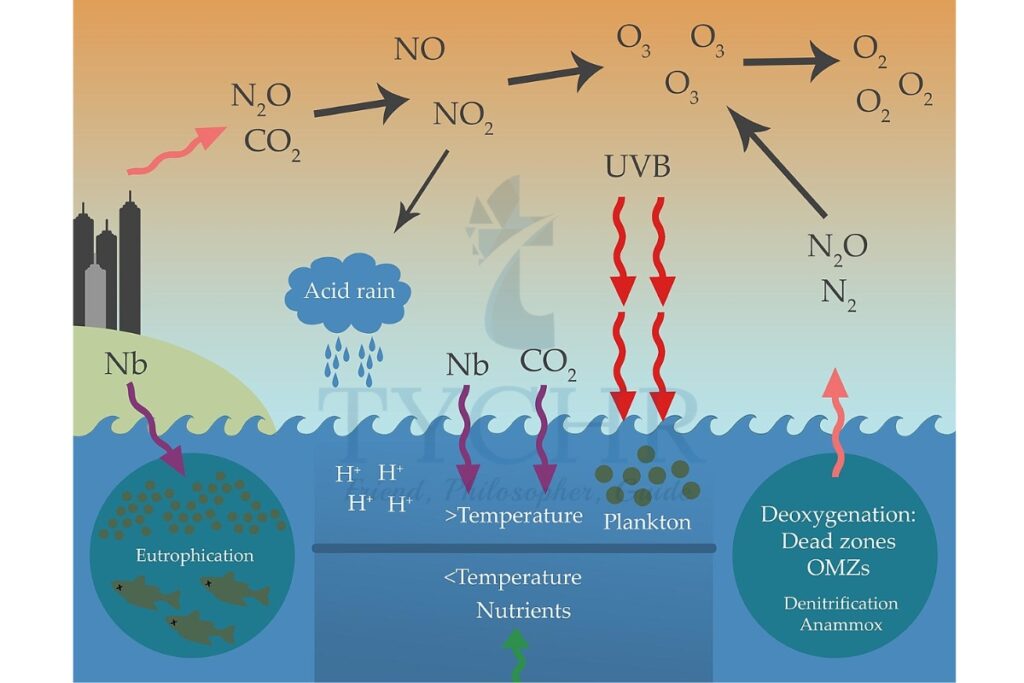
- Secondary pollutant- Tropospheric ozone—or ground-level ozone is formed by reactions involving oxides of nitrogen (NOx).
Nitrogen monoxide reacts with oxygen in the presence of sunlight to form nitrogen dioxide (NO2). This is a brown gas that contributes to urban haze. It can also absorb sunlight and break up to release oxygen atoms that combine with oxygen in the air to form ozone.

6.3.2 The Effects of Tropospheric Ozone
- Effect on Forests and Crops
- Interferes with the ability of sensitive plants to produce and store food
- Damages the leaves of trees and other plants, harming the appearance of vegetation in urban areas, national parks, and recreation areas.
- Effect on Humans
- Ozone can harm lung tissues, impair
the body’s defence mechanism, increase - Respiratory tract infections, and aggravate asthma, bronchitis, and pneumonia.
- Even at relatively low levels, coughing, choking, and sickness increase.
- The long-term effects include premature ageing of the lungs.
- Children born and raised in areas where there are high levels of ozone can experience up to a 15 per cent reduction in their lung capacity.
- Ozone can harm lung tissues, impair
- Effects on Materials
High levels of ozone can damage fabrics and rubber materials. - Ozone and Smog Photochemical smog is associated with certain climates – in particular, high air pressure systems. This is because winds in a high-pressure system are usually weak. Hence pollutants remain in the area and are not dispersed.
6.3.3 Pollution Management Strategies
- Reduction in the burning of fossil fuels
- Greater use of energy-efficient technologies such as hybrid/electric cars
- Increased use of public transport rather than use of private cars
- Car pooling schemes
- Increased use of bicycles or walking
- Use of catalytic convertors to reduce emissions of NOx
- Greater enforcement of emissions standards
- Clean-up measures such as reforestation, re-greening, conservation areas
- Public information regarding air quality.
6.4 Acid Deposition
6.4.1 The Formation of Acid Deposition
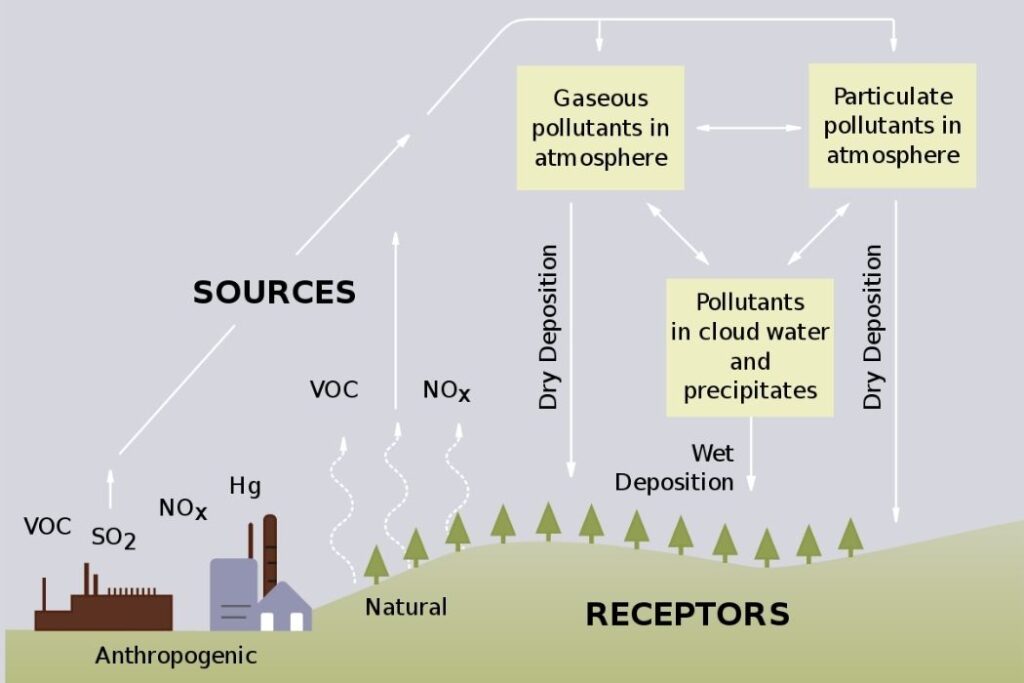
Acid deposition is the increased acidity of rainfall and dry deposition. This is largely as a result of human activity and is caused by carbon dioxide in the atmosphere combining with moisture in the atmosphere.
The major causes of acid rain are:
- The sulphur dioxide and nitrogen oxides produced when fossil fuels are burned.
- Sulphur dioxide and nitrogen oxides are released into the atmosphere. There they are absorbed by the moisture and become weak sulfuric and nitric acids.
- The pH can be as low as 3.
- Dry deposition typically occurs close to the source of emission and causes damage to buildings and structures.
- Wet deposition occurs when the acids are dissolved in precipitation and fall at great distances from the sources.
6.4.2 Direct Effects of Acid Rain
- When levels of acidity increase, many nutrients become unavailable to plants. These include nitrogen, phosphorus, molybdenum, and boron.
- Essential nutrients such as calcium and magnesium can be leached from a soil as it becomes more acidic, and this can be detrimental to plant growth.
- Other nutrients become more common and can be toxic to living organisms. Copper becomes more available in acidic soils. Iron and aluminium may be mobilised when soil pH becomes lower than 4.5.
Impact on Coniferous Trees | Impact on Living Organisms | Impact on Water |
|
|
|
6.4.3 Distribution of Acid Depositio
- Areas producing the acid deposition are not the same as the regions receiving it. Coal fired power stations and heavy concentrations of vehicles emit vast quantities of sulphur dioxide and nitrogen dioxide.
- The main areas experiencing acid rain are those areas downwind of major industrial regions.
- Areas that are currently causing acidification include China and India. This is because both countries burn vast amounts of coal.
- Areas experiencing acidification usually have high rainfall and thin soils. Many of them have forests and lakes.
- Some environments are able to neutralise the effects of acid rain. This is called the buffering capacity. Chalk and limestone areas are very alkaline and can neutralise acids effectively.
6.4.4 Pollution Management Strategies
Altering Human Activity | Controlling the Release of Pollutants |
The first main type of strategy is to alter human activity and to reduce the production of pollutants. The most effective long-term treatment is to reduce the emissions of SOx and NOx. This can be achieved in a variety of ways, such as by:
| The second method is to control the release of pollutants. Methods to achieve this include:
|

