Atomic Nuclear and Particle Physics Theory Notes
Discrete energy and interaction of matter with radiation
Experiments revealing the structure of atom:
J.J. Thomson discovered the existence of electrons. He proposed that the atom is made up of electrons distributed all over the positive charge like that of seeds in a watermelon.
Rutherford experiments involved bombarding alpha particles onto a thin gold sheet. Most of the alpha particles passed straight through the gold foil while a very small portion of alpha particles got reflected and some came back in the direction from which they were fired. From this, the structure of atoms was understood as a small nucleus of positive charge around which electrons revolved.
Light is quantized. It travels in the form of small packets of energy called photons, the energy of each packet being . h is the Planck’s constant and f is the frequency of light.
Atomic spectrum is the series of wavelengths of light released when a gas at low pressure is subjected to high voltages. This spectrum is called emission spectrum in particular. This spectrum is unique for a gas. The energy of each photon in this spectrum corresponds to the difference of energy levels the electrons in the atom can occupy.
Δ𝐸 = ℎ𝜈
Photoelectric effect describes the phenomenon of electrons ejected out of the metal when a light of certain minimum frequency is incident on the metal. Einstein’s photoelectric equation describes the relation between the frequency of incident light (𝜈), threshold frequency of the metal (𝜈0) and the maximum kinetic energy of emitted electron. This is given by
𝐾. 𝐸𝑚𝑎𝑥 = ℎ𝜈 − ℎ𝜈0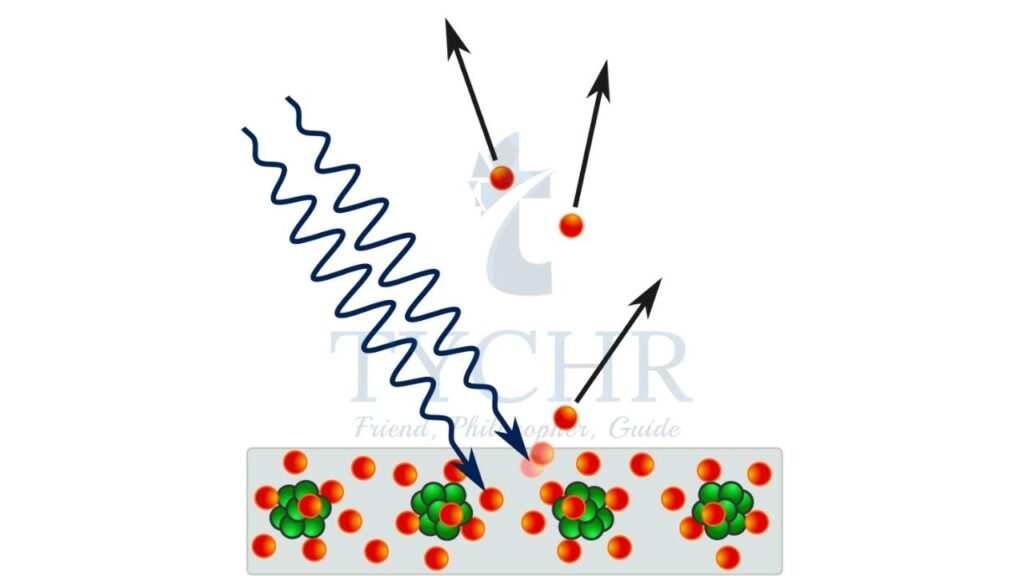
Absorption spectrum is similar to emission spectrum except the energy is absorbed in place of emission by the gas. This gives out black lines in the spectrum of light corresponding to the frequency of the light absorbed. The wavelengths of light emitted in the emission spectrum of an element is the same series of wavelengths obtained in the absorption spectrum.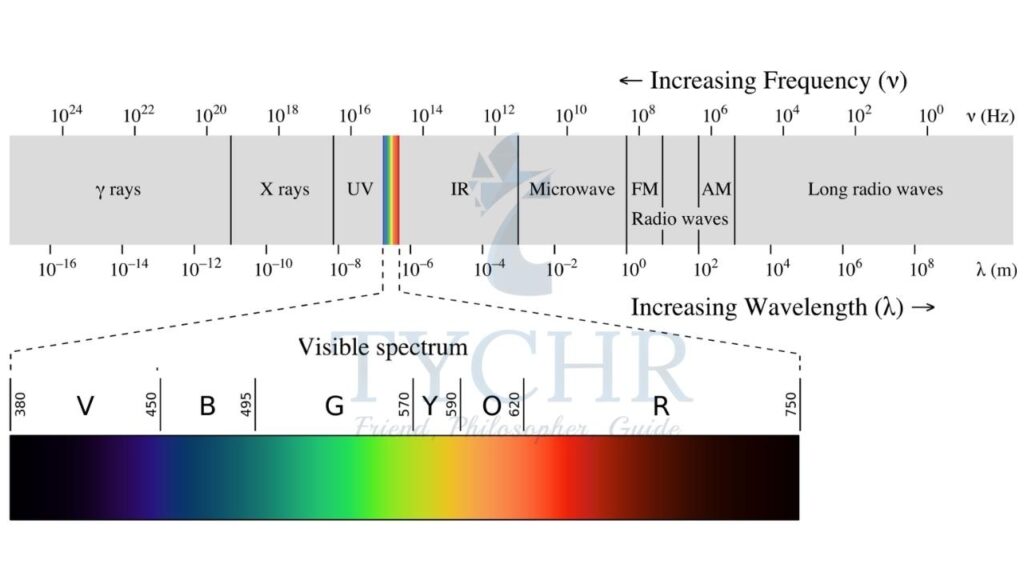
Bohr’s model of atoms revolves around the assumption that an electron does not emit any radiation when it revolves in orbits of certain radius. It emits radiation when it moves from an orbit of higher energy level to an orbit of lower energy level and absorbs radiation when the opposite happens.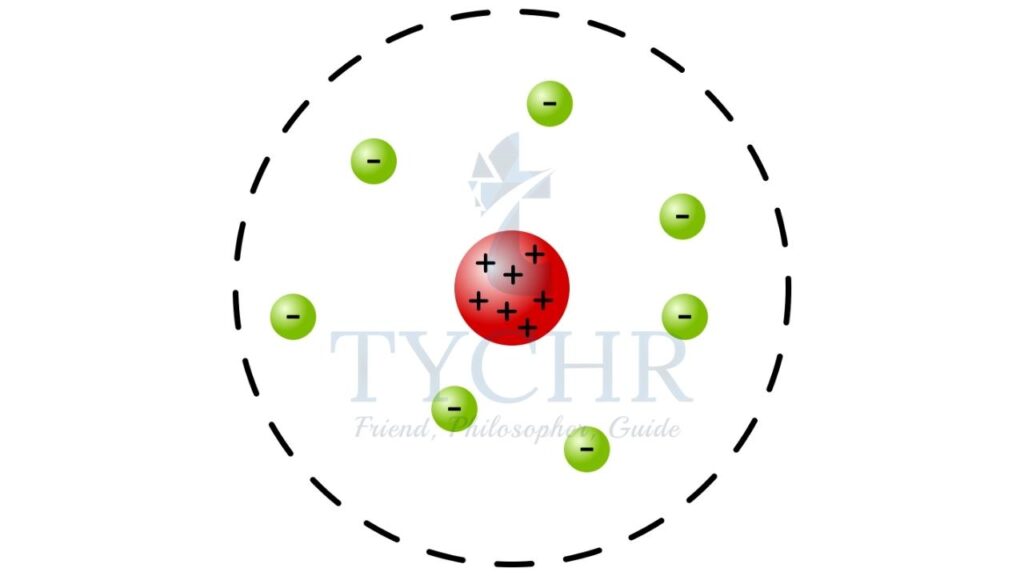
In a hydrogen atom,
The radii of all allowed orbits are given by 𝑟𝑛 =𝜖0𝑛2ℎ2/𝜋𝑚𝑒2
The energy of an electron in an nth is given by: ![]()
De Broglie’s hypothesis explains the dual nature of matter-matter exhibits wave-like nature. For a particle moving with a momentum p, the wavelength of the wave-nature of the particle is given by 𝜆 =ℎ/𝑝
So, every particle exhibits all phenomena a wave exhibits-diffraction, interference among others. But they are not prominent because of small wavelength of large particles. These phenomena can be observed easily in particles of small mass like that of electrons.
Heisenberg’s uncertainty principle puts a limit to the theoretical resolution for the measurement of position and momentum simultaneously. For a particle of momentum P at a position x,
ΔPΔ𝑥 ≥ ℎ/4𝜋
Similarly, for energy and time,
ΔEΔt ≥ ℎ/4𝜋
Schrodinger’s equation describes the probability of finding an electron in a region. The equation describing the probability is represented by 𝜓.
Electrons spin about their own axis. This is represented by + 1/2 and – 1/2 for the two possibilities of spin.
Mass-energy equivalence described by Einstein establishes a bridge between matter and energy. The mass and energy are equivalent and can be represented by 𝐸 = 𝑚𝑐2
Electron tunnelling states that an electron can move out of a potential barrier (a wall) whose probability increases as the potential barrier becomes smaller (wall becomes thinner).
Properties of the nucleus and radioactivity
Charge and mass:
The charge of the nucleus is the total charge of the protons in the nucleus. The mass of an atom is approximately the sum of the mass of neutrons and protons in the nucleus. The mass of a neutron is approximately the same as that of a proton.
Mass number (A) = Nucleon number = number of protons + number of neutrons
Atomic number (Z) = number of protons
Neutron number (N) = number of neutrons (A-Z)
Radius of nucleus:
Radius of nucleus is given 𝑅 = 𝑅0𝐴1/3.
Which gives the volume to be 𝑉 = 𝑉0A. This means that the density of all nuclei is same.
Binding energy:
Binding energy is the energy released when the nucleus is put together from its constituent protons and neutrons. It is also equal to the energy to be supplied to the nucleus to separate it into its constituent nucleons. The change in mass when the nucleus when the nucleus forms from its constituent protons and neutrons is called mass defect. The mass defect and binding energy are related by mass-energy equivalence.
𝐵. 𝐸 = Δ𝑚𝑐2
The binding energy released per nucleon determines the stability of neutron. Higher the binding energy per neutron, higher the stability. In the periodic table, this value is highest around atoms of Iron, making them the most stable.
Atomic unit of mass and electronvolt are related as 1u=931.494MeV.
Types of radioactive decay:
Alpha: Alpha radiation is helium nucleus. Atoms of higher atomic mass release helium nucleus to become more stable.
88𝑅𝑎 226 → 86𝑅𝑛 222 + 2𝐻𝑒4
Beta +: A proton converts into a neutron releasing a positron.
11𝑁𝑎22 → 10𝑁𝑒22 + 𝑒+ + v
Beta -: A neutron converts into a proton releasing an electron.
14C6 → 14𝑁7 + 𝑒– + v–
Gamma: A nucleus falls to a lower energy level releasing energy.
Decay rate:
In a radioactive decay, the number of nuclei (N) remaining after a time t when the initial nuclei are N0 is given by 𝑁 = 𝑁0𝑒−𝜆t
𝜆 is called the decay constant.
Half life is the time taken for the number of nuclei to fall to half the initial number.
𝑡1/2 = 0.693/𝜆
Activity is the rate of decay given by 𝑑𝑁/𝑑𝑡 = 𝐴0𝑒−𝜆𝑡.
E.g.: Cobalt-60 has a half-life of 5 years. If the initial decay rate is 40 beta particles per second, find the rate after 15 years.
Ans. After 5 years the rate is 20 beta particles per second. After 10 years the rate is 10 beta particles per second. After 15 years the rate is 5 beta particles per second.
Background radiation:
It is the radioactive noise emitted by objects in a place which interferes with the results of an experiment. When a Geiger-Muller tube is left in a room, it detects the radiation in the room and this can be corrected from the results of the experiment.
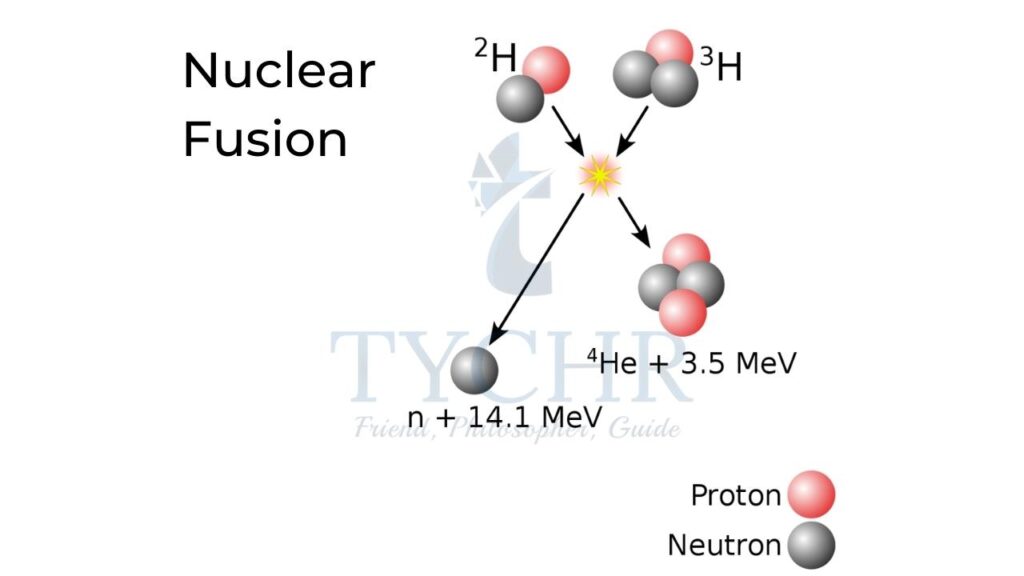
Nuclear fusion:
It is the fusion of two nuclei to form a bigger one.
E.g.: Calculate the energy released in the following reaction.
1𝐻2 + 1𝐻2 → 2𝐻𝑒3 + 0n1
Ans. The difference in masses of products and reactants is 0.18883u. So, the energy released is 17.6MeV.
Nuclear fission:
A nucleus splits into two stable nuclei.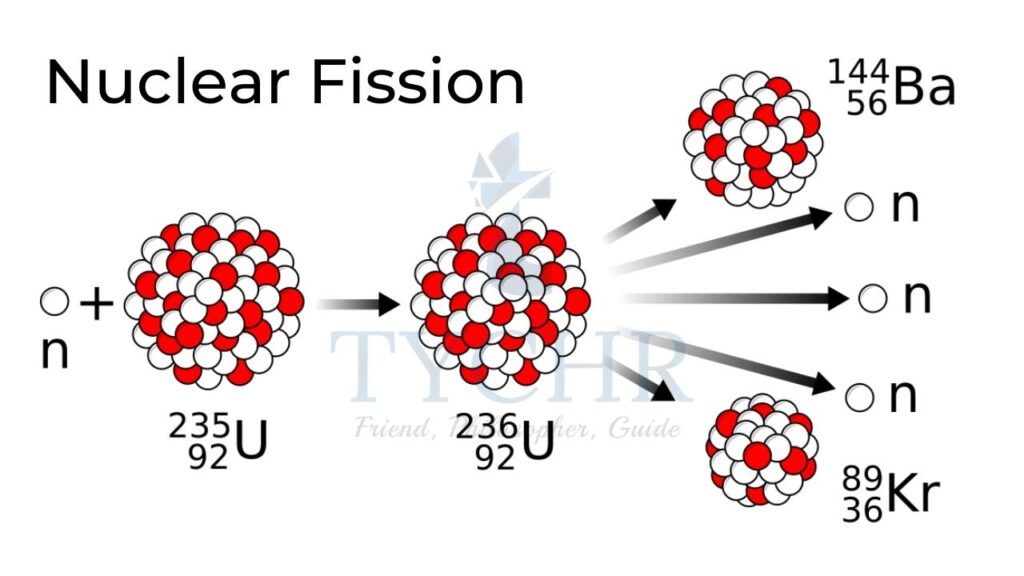
The structure of matter
Classifying particles:
Classifying based on interactions between particles.
Protons and electrons having charge, take part in electromagnetic interactions.
Protons and neutrons being nucleons, take part in nuclear interactions.
Neutrino takes part in weak interactions.
Classifying based on mass.
Hadrons are heavy particles that take part in nuclear interactions. Protons and neutrons fall in this category.
Leptons are light particles. Electrons and neutrinos fall in this category.
Photons have no mass and are categorised as exchange bosons.
Exchange forces:
In 1933, Hideki Yukawa developed the theory of exchange forces which describes force as an interaction which involves exchange of particles. If the force is repulsive, the photon is released by one of particle towards the other particle and it is received by the particle. If the force is attractive, the particles are released away from the other particle and travels like a boomerang.
The photons released follow the Heisenberg’s uncertainty principle Δ𝐸Δ𝑡 ≥ℎ/4𝜋. Where Δ𝐸 is the energy of photon and𝐸Δ𝑡 is the time for which the photon travels.
In electromagnetic interactions, photons are the exchange particles.
In nuclear interactions, high energy particles which have a short span called pions are exchange particles.
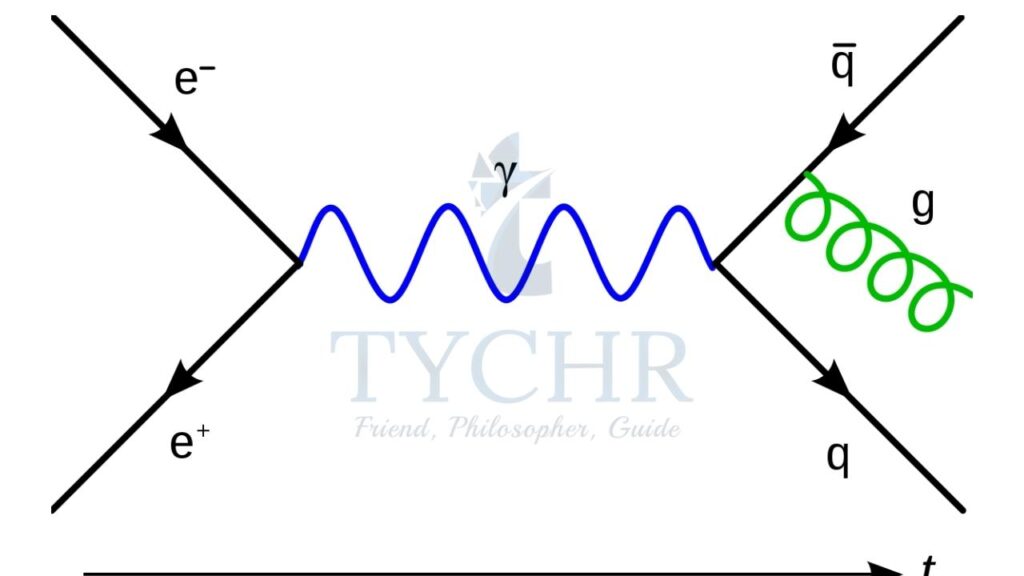
Feynman diagrams:
Feynman diagrams represent the exchange particle theory in a diagrammatic manner.
- The exchange particles are represented with wavy lines and particles with straight lines.
- Vertices representing interaction consist of two particles – one entering and one leaving and an exchange particle.
- Time progresses from left to right.
- Particles have arrows pointing forward in time and anti-particles backwards.
Baryons and mesons:
Mesons take part in nuclear interactions and hence fall under hadrons.
Hadrons can now be categorized into two groups.
- Baryons: Protons etc
- Mesons: Pions etc
Conservation principles:
Particles are assigned baryon and lepton numbers which are also conserved along with charge in nuclear reactions.
Spin is similar to electron spin in an atom which can be either 1/2 or 1(1/2) for baryons and 0 or 1 for mesons.
Strangeness is another quantum number which is not conserved in weak interactions.
All hadrons are made out of 6 flavours of quarks and 6 antiquarks. Each quark has a spin 1/2. So, mesons with spin 1 or 0 have 2 quarks and baryons with spin 1/2 or 1(1/2) have 3 quarks. Strangeness number is the number of strange quarks in the particle (+1 for a strange and -1 for an anti-strange).
Quark confinement: Quarks are confined by very strong attractive forces. When they are forced to be pulled apart, the energy supplied would be so high that a new quark or anti quark is formed.
Colour force:
Electric charge is two sided. So, it is assigned two signs – positive and negative. Quarks are assigned six colours which in right combination give white light. Anti-quarks are given anti colours.
Gluons are the exchange particles of colour force. Gluons have a combination of colour and anti-colour. The quarks change colour upon releasing and absorbing a gluon. A blue quark releases a green anti-blue gluon and changes to green. A blue quark absorbs this gluon and changes to green.
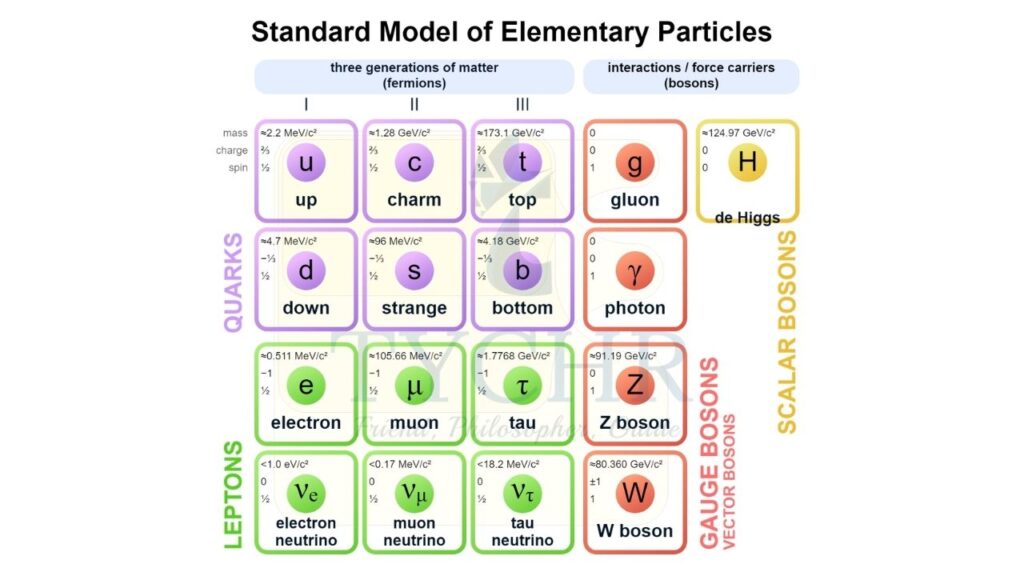
The standard model:
The standard model divides particles into 2 groups.
- Fermions-quarks and leptons
- Bosons-exchange particles for fundamental forces
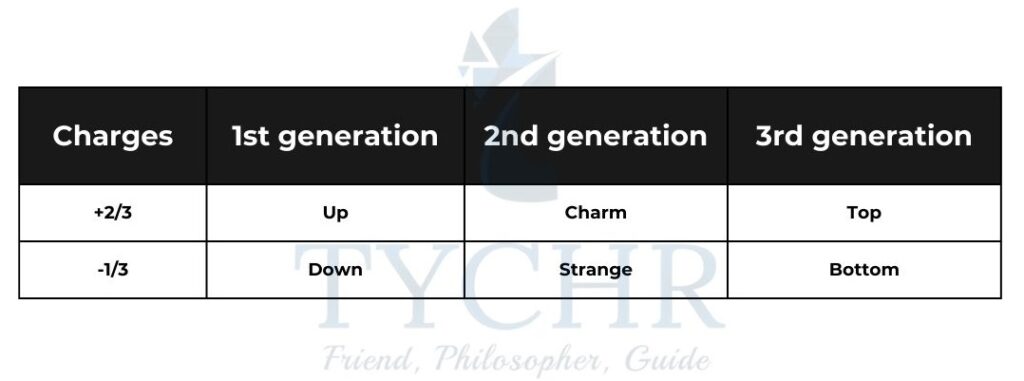
Quarks:
Quarks are arranged into 3 generations according to mass. Each generation contains a +2/3 and -1/3 quark. When the flavour of a quark changes, the change in charge should always be 1e.
Leptons:
There are 6 types of leptons. These are arranged into 3 generations each of which are assigned lepton numbers. Leptons can interact with leptons of the same generation.
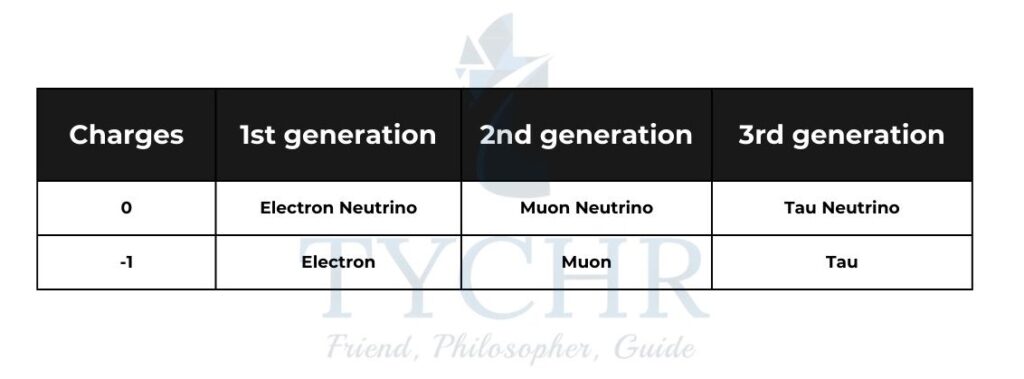
Bosons:
The three fundamental forces each have an associated boson. There are three types of bosons involved with weak force, W+ and W– when there is exchange of charge and Z0 when there is no exchange of charge.
Higgs boson:
The W and Z bosons have no mass and are associated with a field known as the Higgs field and the associated particle of this field is the Higgs boson.