IB Chemistry HL Paper 3 Question Bank
If you’re looking for a comprehensive question bank for IB Chemistry HL Paper 3, you’ve come to the right place. This extensive question bank covers all of the content from the HL syllabus, so you can be sure you’re thoroughly prepared for your exams. The question bank is updated regularly, so you’ll always have access to the most up-to-date information. And if you need any help, our team of experts are always on hand to answer your queries.
Time: 1 hour 15 minutes
Instructions to candidates
- Answers must be written within the answer boxes provided.
- A calculator is required for this paper.
- A clean copy of the chemistry data booklet is required for this paper.
- The maximum mark for this examination paper is [45 marks].
SECTION A
1.) Powdered zinc was reacted with 25 cm3 of 1.000 mol/dm3 copper (II) solution in an insulated beaker. The graph below shows the temperature plotted against time
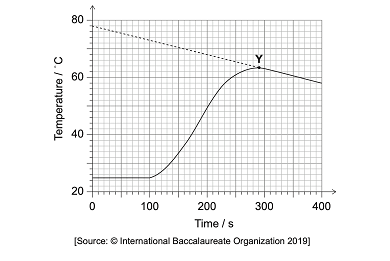
a.) Estimate the time when powdered zinc was placed in the beaker using the graph above
Answer
100 seconds because that’s the data point when the graph begins to increase and slope. It shows that a reaction is occurring.
b.) State what Y indicates in the graph
Answer
Y is the maximum temperature attained in the reaction. According to the graph, the temperature is 73 degree celsius.
c.) The experiment was carried out multiple times to determine the enthalpy. The volume and concentration of copper(II) sulfate was kept constant but the mass of zinc was varied during each trial. Suggest if zinc or copper(II) sulfate should be in excess for each trial.
Answer
Since the amount of copper (II) sulfate is constant and zinc is in excess, it will not yield different results in each trial.
2.) Bromine and methanoic acid react in an aqueous solution. The equation is shown below:
Br2 (aq) + HCOOH (aq) → 2Br– (aq) + 2H+ (aq) + Br2 (g)
The reaction was monitored by plotting a graph of the volume of carbon dioxide produced against time.
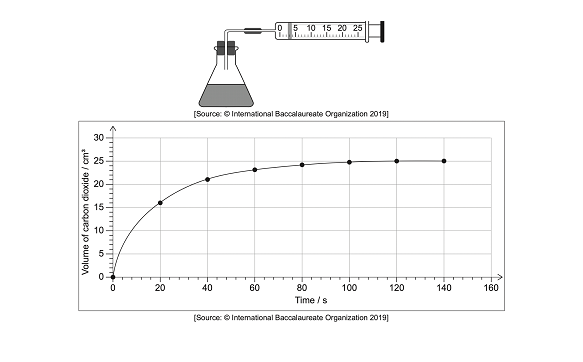
a.) Determine the rate of reaction at 20 seconds using the graph
Answer
A tangent line is drawn at 20 seconds and the gradient of the line is determined. The slope of the line is 0.35 cm3/s
b.) Suggest another variable that can be measured to determine the rate of reaction
Answer
Pressure or mass of gas since gas is produced in the reaction. An alternative is the color of the solution since Bromine (reactant) is coloured. However, the Bromine ion is not.
c.) There are random errors and systematic errors.
i.) One such systematic error is associated with the use of the gas syringe. Outline what this error is and how it affects the calculated rate of reaction.
Answer
Gas could leak out of the syringe so the rate of reaction calculated would essentially be lower.
ii.) State one error that could possibly occur from using an accurate stopwatch
Answer
However accurate the stopwatch is, human reaction time will always be accounted as human error.
3.)
a.)
i.) Explain the difference between crystalline and amorphous materials
Answer
Crystalline materials have a well-defined, repeating atomic arrangement in three dimensions, whereas amorphous materials lack long-range order and have a disordered atomic arrangement.
ii.) A sample of a crystalline material has a density of 2.70 g/cm3. The unit cell of the crystal contains two atoms, and its edge length is 5.08 Å. Calculate the molar mass of the material
Answer
The volume of the unit cell of the crystal can be calculated as (5.08 Å)3 = 131 Å3.) Since there are two atoms per unit cell, the density of the material can be calculated as mass/volume = 2 x molar mass/(6.022 x 1023 x 131 x 10-24) = 2.70 g/cm3.) Solving for the molar mass gives 59.7 g/mol.
4.)
a.)
i.) Describe the bonding in silicon dioxide and how it influences the structure of the material.
Answer
Silicon dioxide has a covalent network bonding, where each silicon atom is covalently bonded to four oxygen atoms, and each oxygen atom is covalently bonded to two silicon atoms. This results in a tetrahedral structure where each silicon atom is surrounded by four oxygen atoms, and each oxygen atom is surrounded by two silicon atoms.
ii.) Explain how the presence of a dopant can change the electrical properties of a semiconductor.
Answer
Doping involves intentionally introducing impurity atoms into a semiconductor, which can change its electrical properties. Adding impurity atoms with more valence electrons than the semiconductor atoms creates “extra” electrons in the conduction band, which can also allow the material to conduct electricity more easily.
OPTION B – Biochemistry
1.) This question is about Alcohol Dehydrogenase (ADH) Catalysis
a.) Explain the role of Alcohol Dehydrogenase (ADH) in the metabolism of ethanol, including the oxidation and reduction reactions involved.
Answer
Alcohol Dehydrogenase (ADH) is an enzyme that catalyzes the reversible oxidation of ethanol to acetaldehyde, using nicotinamide adenine dinucleotide (NAD+) as a cofactor. The overall reaction can be represented as follows:
CH3CH2OH + NAD+ ↔ CH3CHO + NADH + H+
b.) Using the data in the data booklet, compare the catalytic efficiency of ADH from different organisms, and explain any observed differences.
Answer
The catalytic efficiency of ADH can be compared by calculating the turnover number (kcat) and the Michaelis-Menten constant (Km) for ADH from different organisms. For example, ADH from yeast has a higher kcat than ADH from humans, but a lower affinity for ethanol (higher Km value). This suggests that yeast ADH is optimized for fast ethanol metabolism, while human ADH is optimized for efficient use of NAD+.
2.)
a.) Define the term “lipid bilayer.” What is the role of cholesterol in the lipid bilayer?
Answer
The lipid bilayer is a double layer of phospholipid molecules that forms the outer membrane of cells. Cholesterol is a type of lipid that is found in the lipid bilayer, where it helps to stabilize the membrane and regulate its fluidity.
b.) Draw the structure of the amino acid glycine and explain its role in protein structure.
Answer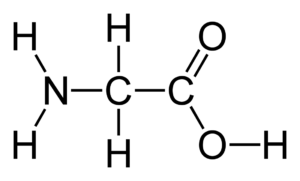
Glycine is an amino acid that does not have a side chain, which makes it more flexible than other amino acids. Glycine is often found in flexible regions of proteins, such as in the turns that connect different parts of the protein structure.
3.) This question is about Chiral Molecules
a.) Define the term “chiral molecule.” What is the significance of chirality in medicinal chemistry?
Answer
A chiral molecule is a molecule that has a non-superimposable mirror image, known as an enantiomer. Chirality is significant in medicinal chemistry because enantiomers can have different pharmacological properties, including differences in efficacy, potency, and toxicity.
b.) Draw the structure of the amino acid cysteine and explain its role in protein structure.
Answer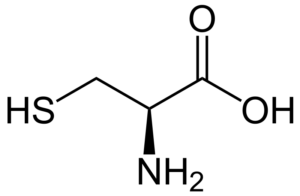
Cysteine is an amino acid that contains a sulfur atom, which can form disulfide bonds with other cysteine residues in proteins. These disulfide bonds help to stabilize the protein structure.
4.)
a.) Explain absorption spectrum in relation to photosynthesis.
Answer
An absorption spectrum is a graph showing the wavelengths of light that are absorbed by a substance, such as a photosynthetic pigment. In photosynthesis, an absorption spectrum shows the wavelengths of light that are absorbed by chlorophyll and other pigments in the light-harvesting complex of a plant.
b.) Using a graph of an absorption spectrum, explain the relationship between light wavelength and the rate of photosynthesis.
Answer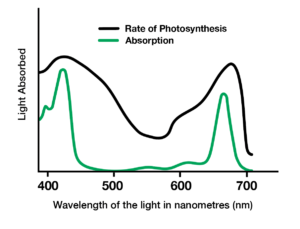
The graph of an absorption spectrum typically shows a peak at the wavelength(s) of light most efficiently absorbed by the pigment. In the case of photosynthesis, the peak is typically around 400-500 nm (blue light) and 650-700 nm (red light). The rate of photosynthesis is highest at these wavelengths because they correspond to the wavelengths of light most efficiently used by chlorophyll for photosynthesis.
5.)
a.) Discuss the potential benefits and drawbacks of genetically modifying crops for increased yield.
Answer
Benefits of genetically modifying crops for increased yield include increased food production to meet the needs of a growing population, resistance to pests and disease, and increased drought tolerance. Drawbacks include the potential for unintended environmental consequences, such as the spread of modified genes to non-target species, and concerns over the safety of consuming genetically modified foods.
b.) How might advances in genetic engineering technology impact the future of agriculture?
Answer
Advances in genetic engineering technology have the potential to revolutionize agriculture by enabling the development of crops with desirable traits, such as increased yield, improved nutritional value, and resistance to pests and disease.
6.)
a.)
i) Define the term “allosteric enzyme”
Answer
An allosteric enzyme is a type of enzyme that changes its shape and activity in response to the binding of a molecule (the allosteric effector) to a site on the enzyme that is distinct from the active site.
ii.) Explain the role of allosteric enzymes in metabolic pathways
Answer
Allosteric enzymes play a crucial role in metabolic pathways by regulating the rate of reaction of the pathway. The binding of the allosteric effector to the enzyme can either activate or inhibit the enzyme’s activity, which in turn affects the rate of reaction of the pathway. This allows cells to control and regulate their metabolism in response to changing conditions.
OPTION C – Energy
1.) This question is about Carbon Capture and Energy
a.) Describe the chemical reactions involved in the absorption and release of carbon dioxide by amine solvents.
Answer:
When carbon dioxide reacts with amine solvents, it forms a weak acid that can be easily regenerated by heating the solution. The overall reaction can be written as follows: CO2 + 2NH3 + H2O → (NH4)2CO3.
b.) Give an example of a real-world application of carbon capture and storage technology. How is this technology being implemented and what are the expected benefits?
Answer:
One real-world application of CCS technology is the Boundary Dam Power Station in Saskatchewan, Canada.) This power station captures carbon dioxide emissions from coal combustion and stores them underground in a nearby oil field. The expected benefits of this technology include a reduction in greenhouse gas emissions and the production of cleaner energy.
2.)
a.) What is Hess’s Law? How can it be used to calculate the enthalpy change of a reaction?
Answer:
Hess’s Law states that the enthalpy change of a reaction is independent of the pathway taken, as long as the initial and final states are the same. It can be used to calculate the enthalpy change of a reaction by summing the enthalpy changes of a series of reactions that add up to the desired reaction.
b.) Draw a potential energy diagram for an exothermic reaction. Label the activation energy and the enthalpy change.
Answer: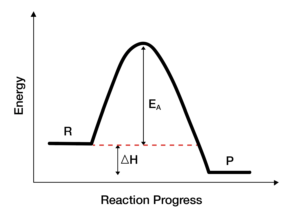
c.) Explain how temperature affects the spontaneity of a reaction.
Answer:
Increasing the temperature of a reaction can increase the entropy of the system, making it more likely to be spontaneous.
3.) This question is about Hydrogen Fuel Cell
a.) Define the term “hydrogen fuel cell.” Describe how a hydrogen fuel cell works.
Answer:
A hydrogen fuel cell is an electrochemical device that converts hydrogen and oxygen into water, producing electricity in the process. In a hydrogen fuel cell, hydrogen gas is supplied to the anode of the fuel cell, where it is split into protons and electrons. The protons move through an electrolyte membrane to the cathode, while the electrons flow through an external circuit, producing electricity. At the cathode, the protons and electrons combine with oxygen to form water.
b.) Discuss the potential environmental impacts of large-scale hydrogen fuel cell production. What are some strategies that could be used to minimize these impacts?
Answer:
Large-scale hydrogen fuel cell production could have potential environmental impacts, such as the production of greenhouse gasses during hydrogen production and the potential for water scarcity. Strategies to minimize these impacts include the use of renewable energy sources to produce hydrogen, such as solar or wind power, and the use of water-efficient hydrogen production technologies, such as electrolysis.
4.)
a.) Explain the concept of bond dissociation enthalpy and discuss how it varies with bond order and bond length.
Answer:
Bond dissociation enthalpy is the energy required to break a particular bond in one mole of gaseous molecules under standard conditions. It varies with bond order and bond length. Generally, the bond dissociation enthalpy increases with bond order because stronger bonds require more energy to break. Bond dissociation enthalpy also increases with bond length because longer bonds are weaker and easier to break.
b.) Using the data booklet, calculate the average bond dissociation enthalpy for the O-H bond in water.
Answer:
Using the given data, the average bond dissociation enthalpy for the O-H bond in water can be calculated as follows:
ΔH°(O-H) = 2ΔH°(O-H) = 2 × 463 kJ mol-1 = 926 kJ mol-1
c.) Draw the Lewis structure and predict the molecular geometry of the NO2+ ion. Use this information to explain why the N-O bond length in NO2+ is shorter than in NO2–.
Answer:
The Lewis structure of NO2+ has one less electron than the neutral molecule NO2.) Therefore, the ion has a linear molecular geometry with a N-O double bond and a N=O bond. 2+ is shorter than in NO2- because the NO2+ ion has one less electron than the NO2- ion. The positive charge on the NO2+ ion causes the electron density to shift away from the N atom, making the N-O bond shorter and stronger.
5.)
a.) Given the following data for the reaction between enzyme X and substrate Y:
[X] (M) | [Y] (M) | Initial rate (µM/s) |
0.10 | 0.10 | 0.18 |
0.20 | 0.10 | 0.36 |
0.20 | 0.20 | 0.72 |
Calculate the rate constant for the reaction and determine the order of the reaction with respect to both enzyme X and substrate Y.
Answer:
To calculate the rate constant, we can use the equation: rate = k[X]a[Y]b, where k is the rate constant and a and b are the orders of the reaction with respect to enzyme X and substrate Y, respectively. By comparing the initial rates for the different concentrations of X and Y, we can see that the reaction is first-order with respect to both X and Y. Using the data for the first set of conditions ([X] = 0.10 M, [Y] = 0.10 M, rate = 0.18 µM/s), we can solve for the rate constant as follows:
0.18 = k(0.10)1(0.10)
k = 1800 M-2s-1
Therefore, the rate constant for the reaction is 1800 M-2s-1, and the order of the reaction with respect to enzyme X and substrate Y is both first-order.
b) A patient with a genetic deficiency in an enzyme involved in glycolysis presents with symptoms of muscle weakness and fatigue. Using the knowledge of glycolysis, describe the potential biochemical causes of these symptoms .
Answer:
The symptoms of muscle weakness and fatigue in the patient with a deficiency in glycolysis could be caused by a decrease in ATP production due to the impaired glycolytic pathway. Specifically, the inability to produce enough ATP through glycolysis could lead to a decrease in muscle contraction and an increase in muscle fatigue.
OPTION D – Medicinal Chemistry
1.)
a.) Define the term ‘drug resistance’
Answer:
Drug resistance refers to the ability of microorganisms or cancer cells to survive and proliferate in the presence of drugs that would normally kill or inhibit them.
b.) Explain how multidrug resistance (MDR) develops in cancer cells and how it can be overcome
Answer:
Multidrug resistance (MDR) develops in cancer cells when they overexpress membrane transporters, such as P-glycoprotein, that can pump out drugs from the cell before they can exert their pharmacological effect. MDR can be overcome by using combination therapy with drugs that have different mechanisms of action or by co-administering drugs that can inhibit the efflux pump.
2.)
a.) Discuss the use of nanoparticles in targeted drug delivery
Answer:
Nanoparticles are submicron-sized particles that can be engineered to encapsulate drugs and target specific tissues or cells. Advantages of using nanoparticles in targeted drug delivery include improved drug solubility and stability, increased drug loading capacity, reduced toxicity and side effects, and enhanced cellular uptake and retention.
b.) Describe the pharmacological properties and therapeutic uses of cannabinoids
Answer:
The pharmacological properties of cannabinoids are mainly mediated by two receptors, CB1 and CB2, which are found in different tissues and cell types. Cannabinoids have been used therapeutically for pain relief, anti-inflammatory effects, anti-emetic effects, and as a treatment for epilepsy and multiple sclerosis. However, the use of cannabinoids is associated with several adverse effects, such as cognitive impairment, cardiovascular effects, and addiction potential.
3.)
a.)
i.) Define the term ‘drug’
Answer:
A drug is a chemical substance that alters the structure or function of the body when administered.
ii.) Explain how the potency of a drug is related to its efficacy
Answer:
The potency of a drug refers to the concentration required to achieve a particular effect, whereas efficacy refers to the maximum effect that can be achieved by the drug. Potency and efficacy are related in that a more potent drug will require a lower concentration to achieve the same effect as a less potent drug, but potency alone does not guarantee efficacy.
b.) Discuss the importance of chirality in drug design
Answer:
Chirality is the property of a molecule that is non-superimposable on its mirror image. Many drugs contain chiral centers and the different enantiomers can have different biological effects. In drug design, it is important to consider the stereochemistry of the molecule to optimize its pharmacological properties and minimize side effects.
4.)
a.)
i.) Define the term ‘bioavailability’
Answer:
Bioavailability refers to the fraction of an administered dose of a drug that reaches the systemic circulation unchanged and is available to exert its pharmacological effect.
ii.) Explain how pH partitioning affects the absorption of weakly acidic and weakly basic drugs in the body
Answer:
pH partitioning refers to the distribution of a drug between its ionized and non-ionized forms in different body compartments with different pH values. Weakly acidic drugs are predominantly non-ionized in acidic environments, such as the stomach, and are better absorbed there, while weakly basic drugs are predominantly non-ionized in basic environments, such as the small intestine, and are better absorbed there.
5.)
a.)
i.) Discuss the mechanism of action of statins as cholesterol-lowering drugs.
Answer:
Statins are cholesterol-lowering drugs that inhibit the enzyme HMG-CoA reductase, which is involved in the biosynthesis of cholesterol in the liver. By reducing the production of cholesterol, statins increase the expression of low-density lipoprotein (LDL) receptors on the surface of liver cells, leading to increased clearance of LDL from the blood and a decrease in LDL cholesterol levels.
ii.) Explain the role of enzyme inhibitors in the treatment of HIV infections
Answer:
Enzyme inhibitors are used in the treatment of HIV infections to inhibit the activity of the viral protease or reverse transcriptase enzymes, which are essential for viral replication.
iii.) Describe the mechanism of action of monoclonal antibodies as anticancer drugs.
Answer:
Monoclonal antibodies are laboratory-produced antibodies that can bind specifically to cancer cells and induce an immune response against them. They can target cancer cell surface markers or growth factors and inhibit cell proliferation, induce apoptosis, or activate the immune system.
6.)
a.) Penicillin is an antibiotic used to treat bacterial infections.
i.) Discuss the mechanism of action of penicillin in inhibiting bacterial cell wall synthesis.
Answer:
Penicillin inhibits bacterial cell wall synthesis by irreversibly binding to and inhibiting the enzyme transpeptidase, which is responsible for cross-linking peptidoglycan chains in the cell wall. This leads to weakened cell walls and eventually cell lysis.
ii.) Describe the main types of antibiotic resistance mechanisms and explain how they arise.
Answer:
The main types of antibiotic resistance mechanisms are: enzymatic degradation of the antibiotic, modification of the antibiotic target site, and efflux pumps that actively pump out the antibiotic from the cell. Antibiotic resistance arises through genetic mutations or acquisition of resistance genes from other bacteria through horizontal gene transfer.
7.)
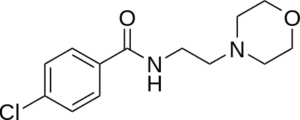
a.) The following structures show four different classes of antidepressants:
1.)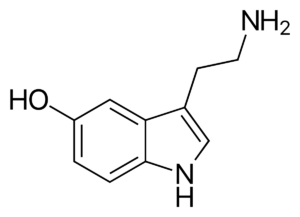 2.)
2.)
3.)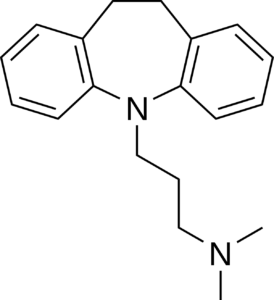 4.)
4.)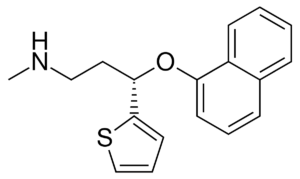
i.) Name each class of antidepressants shown.
Answer:
Class 1: Selective Serotonin Reuptake Inhibitors (SSRIs)
Class 2: Monoamine Oxidase Inhibitors (MAOIs)
Class 3: Tricyclic Antidepressants (TCAs)
Class 4: Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs)
ii.) For each class of antidepressants, explain the mode of action and give an example of a drug in that class.
Answer:
Class 1: SSRIs work by selectively inhibiting the reuptake of serotonin, thereby increasing its concentration in the synaptic cleft. Example drug: fluoxetine (Prozac).
Class 2: MAOIs work by irreversibly inhibiting the enzyme monoamine oxidase, which is responsible for the breakdown of serotonin, norepinephrine, and dopamine. Example drug: phenelzine (Nardil).
Class 3: TCAs work by blocking the reuptake of both serotonin and norepinephrine. Example drug: amitriptyline (Elavil).
Class 4: SNRIs work by selectively inhibiting the reuptake of both serotonin and norepinephrine. Example drug: venlafaxine (Effexor).
SECTION – B
OPTION A – Materials
1.) Lithium metal is obtained by electrolysis of molten lithium chloride.
a.) Calculate the time it takes to deposit 0.842 g of lithium using a current of 3.31 A. Formula is Q (Charge) = I (Current) x T (Time)
Answer
Number of moles = m / mr = 0.842/6.94 = 0.121 moles
Time = Charge / Current = (96500 x 0.121) / 3.31 = 3527.64 seconds → 3530 s
b.) When lithium is doped into graphene, it is seen to have some superconductive properties. However, the Meissner effect is absent. Describe the Meissner effect.
Answer
The expulsion of a magnetic field from the interior of a material that is in the process of becoming a superconductor.
c.) Lithium atoms enhance the phonon binding of electrons in graphene at very low temperatures. This in turn suggests the formation of Cooper Pairs. Outline how they are formed.
Answer
Cooper pairs are formed by electron-phonon interactions – an electron in the cation lattice will distort the lattice around it, creating an area of greater positive charge density around itself.
d.) Lithium forms a crystalline lattice with the unit cell structure shown below. Describe the characteristics of a crystalline lattice structure
Answer
The crystal lattice is the symmetrical three-dimensional structural arrangements of atoms, ions or molecules inside a crystalline solid as points.
2.) Nanotechnology has allowed the manipulation of materials on the atomic level
a.) Describe the structure and bonding of a carbon nanotube
Answer
The chemical bonding of CNTs is composed entirely of sp2 bonds, similar to those of graphite and are arranged in a hexagonal pattern. CNTs naturally align themselves into ropes held together by van der Waals forces.
b.) Suggest one place where carbon nanotubes are used
Answer
They are used to make bullet-proof jackets, aircraft and spacecraft bodies.
c.) Liquid crystals have many applications. A molecule which acts as a chiral nematic thermotropic liquid-crystal is given.
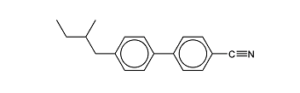
i.) Define thermotropic liquid crystals
Answer
They are anisotropic liquids that possess a mesophase (a phase with crystal and liquid properties) within a certain temperature range. In a spectrometer magnet, LC molecules tend to orient to a common direction which defines the director of the liquid crystal.
ii.) Mark the chiral carbon atom with a cross
Answer
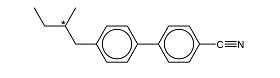
3.) Superconductors has a variety of applications
a.) Define superconductors
Answer
Superconductors are solids that at low temperatures exhibit zero resistance to the flow of electrical current
b.) Differentiate between Type 1 and Type 2 superconductors
Answer
Type-I superconductors are generally pure metals. Type-II superconductors are generally alloys and complex oxides of ceramics. Type I superconductors are those superconductors that lose their superconductivity very easily or abruptly when placed in the external magnetic field whereas Type II superconductors lose their superconductivity gradually but not easily or abruptly when placed in the external magnetic field.
c.) Give two examples of a superconductor
Answer
Some examples of superconductors are aluminum, copper oxide, etc. These substances superconduct at temperatures below the critical temperature.
4.) Heavy metals are known to be toxic even in the lowest of the concentrations
a.) Suggest a reason why heavy metals are toxic
Answer
Heavy metals are toxic because the molecules that make up the metal damage or negatively interact with the cells in your body that are essential to keep your organs functioning.
b.) Outline how heavy metals can be removed from solutions other than precipitation
Answer
Chelation using an EDTA, ion exchange systems, or adsorption by water plants
c.) Chelating agents are used to treat heavy metal poisoning.
1.) State one feature of a chelating agent
Answer
Chelating agents are capable of binding to toxic metal ions to form complex structures which are easily excreted from the body removing them from intracellular spaces.
5.) This question is about polymers
a.) Define thermoplastic polymers and thermosetting polymers
Answer
Thermoplastic polymers are a form of plastic polymers that are moldable at a certain high temperature and solidify when cooled. Thermosetting polymers are polymers consisting of cross-linked structure or heavily branched molecules. It is a permanent setting polymer as it hardens and sets during the molding process and cannot be softened again.
b.) Cyclohexane can be converted to caprolactam using concentrated sulfuric acid as a catalyst. Zeolite can also be used as a catalyst in this reaction, however, they are selective. Suggest why they are selective catalysts.
Answer
Zeolites have cage-like structures in a specific shape and size. Only reactants with an appropriate size will be able to fit inside and react.
c.) Caprolactam reacts with water to form a compound X, a monomer.
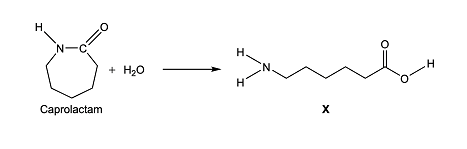
Identify the two terminal functional groups in the compound X.
Answer
Amino and Carboxyl respectively
6.)
a.)
i.) Explain the difference between crystalline and amorphous materials
Answer
Crystalline materials have a well-defined, repeating atomic arrangement in three dimensions, whereas amorphous materials lack long-range order and have a disordered atomic arrangement.
ii.) A sample of a crystalline material has a density of 2.70 g/cm3. The unit cell of the crystal contains two atoms, and its edge length is 5.08 Å. Calculate the molar mass of the material
Answer
The volume of the unit cell of the crystal can be calculated as (5.08 Å)3 = 131 Å3.) Since there are two atoms per unit cell, the density of the material can be calculated as mass/volume = 2 x molar mass/(6.022 x 1023 x 131 x 10-24) = 2.70 g/cm3.) Solving for the molar mass gives 59.7 g/mol.
7.)
a.)
i.) Describe the bonding in silicon dioxide and how it influences the structure of the material.
Answer
Silicon dioxide has a covalent network bonding, where each silicon atom is covalently bonded to four oxygen atoms, and each oxygen atom is covalently bonded to two silicon atoms. This results in a tetrahedral structure where each silicon atom is surrounded by four oxygen atoms, and each oxygen atom is surrounded by two silicon atoms.
ii.) Explain how the presence of a dopant can change the electrical properties of a semiconductor.
Answer
Doping involves intentionally introducing impurity atoms into a semiconductor, which can change its electrical properties. Adding impurity atoms with more valence electrons than the semiconductor atoms creates “extra” electrons in the conduction band, which can also allow the material to conduct electricity more easily.
OPTION B – Biochemistry
1.) This question is about Alcohol Dehydrogenase (ADH) Catalysis
a.) Explain the role of Alcohol Dehydrogenase (ADH) in the metabolism of ethanol, including the oxidation and reduction reactions involved.
Answer
Alcohol Dehydrogenase (ADH) is an enzyme that catalyzes the reversible oxidation of ethanol to acetaldehyde, using nicotinamide adenine dinucleotide (NAD+) as a cofactor. The overall reaction can be represented as follows:
CH3CH2OH + NAD+ ↔ CH3CHO + NADH + H+
b.) Using the data in the data booklet, compare the catalytic efficiency of ADH from different organisms, and explain any observed differences.
Answer
The catalytic efficiency of ADH can be compared by calculating the turnover number (kcat) and the Michaelis-Menten constant (Km) for ADH from different organisms. For example, ADH from yeast has a higher kcat than ADH from humans, but a lower affinity for ethanol (higher Km value). This suggests that yeast ADH is optimized for fast ethanol metabolism, while human ADH is optimized for efficient use of NAD+.
2.)
a.) Define the term “lipid bilayer.” What is the role of cholesterol in the lipid bilayer?
Answer
The lipid bilayer is a double layer of phospholipid molecules that forms the outer membrane of cells. Cholesterol is a type of lipid that is found in the lipid bilayer, where it helps to stabilize the membrane and regulate its fluidity.
b.) Draw the structure of the amino acid glycine and explain its role in protein structure.
Answer
Glycine is an amino acid that does not have a side chain, which makes it more flexible than other amino acids. Glycine is often found in flexible regions of proteins, such as in the turns that connect different parts of the protein structure.
3.) This question is about Chiral Molecules
a.) Define the term “chiral molecule.” What is the significance of chirality in medicinal chemistry?
Answer
A chiral molecule is a molecule that has a non-superimposable mirror image, known as an enantiomer. Chirality is significant in medicinal chemistry because enantiomers can have different pharmacological properties, including differences in efficacy, potency, and toxicity.
b.) Draw the structure of the amino acid cysteine and explain its role in protein structure.
Answer
Cysteine is an amino acid that contains a sulfur atom, which can form disulfide bonds with other cysteine residues in proteins. These disulfide bonds help to stabilize the protein structure.
4.)
a.) Explain absorption spectrum in relation to photosynthesis.
Answer
An absorption spectrum is a graph showing the wavelengths of light that are absorbed by a substance, such as a photosynthetic pigment. In photosynthesis, an absorption spectrum shows the wavelengths of light that are absorbed by chlorophyll and other pigments in the light-harvesting complex of a plant.
b.) Using a graph of an absorption spectrum, explain the relationship between light wavelength and the rate of photosynthesis.
Answer
The graph of an absorption spectrum typically shows a peak at the wavelength(s) of light most efficiently absorbed by the pigment. In the case of photosynthesis, the peak is typically around 400-500 nm (blue light) and 650-700 nm (red light). The rate of photosynthesis is highest at these wavelengths because they correspond to the wavelengths of light most efficiently used by chlorophyll for photosynthesis.
5.)
a.) Discuss the potential benefits and drawbacks of genetically modifying crops for increased yield.
Answer
Benefits of genetically modifying crops for increased yield include increased food production to meet the needs of a growing population, resistance to pests and disease, and increased drought tolerance. Drawbacks include the potential for unintended environmental consequences, such as the spread of modified genes to non-target species, and concerns over the safety of consuming genetically modified foods.
b.) How might advances in genetic engineering technology impact the future of agriculture?
Answer
Advances in genetic engineering technology have the potential to revolutionize agriculture by enabling the development of crops with desirable traits, such as increased yield, improved nutritional value, and resistance to pests and disease.
6.)
a.)
i) Define the term “allosteric enzyme”
Answer
An allosteric enzyme is a type of enzyme that changes its shape and activity in response to the binding of a molecule (the allosteric effector) to a site on the enzyme that is distinct from the active site.
ii.) Explain the role of allosteric enzymes in metabolic pathways
Answer
Allosteric enzymes play a crucial role in metabolic pathways by regulating the rate of reaction of the pathway. The binding of the allosteric effector to the enzyme can either activate or inhibit the enzyme’s activity, which in turn affects the rate of reaction of the pathway. This allows cells to control and regulate their metabolism in response to changing conditions.
OPTION C – Energy
1.) This question is about Carbon Capture and Energy
a.) Describe the chemical reactions involved in the absorption and release of carbon dioxide by amine solvents.
Answer:
When carbon dioxide reacts with amine solvents, it forms a weak acid that can be easily regenerated by heating the solution. The overall reaction can be written as follows: CO2 + 2NH3 + H2O → (NH4)2CO3.
b.) Give an example of a real-world application of carbon capture and storage technology. How is this technology being implemented and what are the expected benefits?
Answer:
One real-world application of CCS technology is the Boundary Dam Power Station in Saskatchewan, Canada.) This power station captures carbon dioxide emissions from coal combustion and stores them underground in a nearby oil field. The expected benefits of this technology include a reduction in greenhouse gas emissions and the production of cleaner energy.
2.)
a.) What is Hess’s Law? How can it be used to calculate the enthalpy change of a reaction?
Answer:
Hess’s Law states that the enthalpy change of a reaction is independent of the pathway taken, as long as the initial and final states are the same. It can be used to calculate the enthalpy change of a reaction by summing the enthalpy changes of a series of reactions that add up to the desired reaction.
b.) Draw a potential energy diagram for an exothermic reaction. Label the activation energy and the enthalpy change.
Answer:
c.) Explain how temperature affects the spontaneity of a reaction.
Answer:
Increasing the temperature of a reaction can increase the entropy of the system, making it more likely to be spontaneous.
3.) This question is about Hydrogen Fuel Cell
a.) Define the term “hydrogen fuel cell.” Describe how a hydrogen fuel cell works.
Answer:
A hydrogen fuel cell is an electrochemical device that converts hydrogen and oxygen into water, producing electricity in the process. In a hydrogen fuel cell, hydrogen gas is supplied to the anode of the fuel cell, where it is split into protons and electrons. The protons move through an electrolyte membrane to the cathode, while the electrons flow through an external circuit, producing electricity. At the cathode, the protons and electrons combine with oxygen to form water.
b.) Discuss the potential environmental impacts of large-scale hydrogen fuel cell production. What are some strategies that could be used to minimize these impacts?
Answer:
Large-scale hydrogen fuel cell production could have potential environmental impacts, such as the production of greenhouse gasses during hydrogen production and the potential for water scarcity. Strategies to minimize these impacts include the use of renewable energy sources to produce hydrogen, such as solar or wind power, and the use of water-efficient hydrogen production technologies, such as electrolysis.
4.)
a.) Explain the concept of bond dissociation enthalpy and discuss how it varies with bond order and bond length.
Answer:
Bond dissociation enthalpy is the energy required to break a particular bond in one mole of gaseous molecules under standard conditions. It varies with bond order and bond length. Generally, the bond dissociation enthalpy increases with bond order because stronger bonds require more energy to break. Bond dissociation enthalpy also increases with bond length because longer bonds are weaker and easier to break.
b.) Using the data booklet, calculate the average bond dissociation enthalpy for the O-H bond in water.
Answer:
Using the given data, the average bond dissociation enthalpy for the O-H bond in water can be calculated as follows:
ΔH°(O-H) = 2ΔH°(O-H) = 2 × 463 kJ mol-1 = 926 kJ mol-1
c.) Draw the Lewis structure and predict the molecular geometry of the NO2+ ion. Use this information to explain why the N-O bond length in NO2+ is shorter than in NO2–.
Answer:
The Lewis structure of NO2+ has one less electron than the neutral molecule NO2.) Therefore, the ion has a linear molecular geometry with a N-O double bond and a N=O bond. 2+ is shorter than in NO2- because the NO2+ ion has one less electron than the NO2- ion. The positive charge on the NO2+ ion causes the electron density to shift away from the N atom, making the N-O bond shorter and stronger.
5.)
a.) Given the following data for the reaction between enzyme X and substrate Y:
[X] (M) | [Y] (M) | Initial rate (µM/s) |
0.10 | 0.10 | 0.18 |
0.20 | 0.10 | 0.36 |
0.20 | 0.20 | 0.72 |
Calculate the rate constant for the reaction and determine the order of the reaction with respect to both enzyme X and substrate Y.
Answer:
To calculate the rate constant, we can use the equation: rate = k[X]a[Y]b, where k is the rate constant and a and b are the orders of the reaction with respect to enzyme X and substrate Y, respectively. By comparing the initial rates for the different concentrations of X and Y, we can see that the reaction is first-order with respect to both X and Y. Using the data for the first set of conditions ([X] = 0.10 M, [Y] = 0.10 M, rate = 0.18 µM/s), we can solve for the rate constant as follows:
0.18 = k(0.10)1(0.10)
k = 1800 M-2s-1
Therefore, the rate constant for the reaction is 1800 M-2s-1, and the order of the reaction with respect to enzyme X and substrate Y is both first-order.
b) A patient with a genetic deficiency in an enzyme involved in glycolysis presents with symptoms of muscle weakness and fatigue. Using the knowledge of glycolysis, describe the potential biochemical causes of these symptoms .
Answer:
The symptoms of muscle weakness and fatigue in the patient with a deficiency in glycolysis could be caused by a decrease in ATP production due to the impaired glycolytic pathway. Specifically, the inability to produce enough ATP through glycolysis could lead to a decrease in muscle contraction and an increase in muscle fatigue.
OPTION D – Medicinal Chemistry
1.)
a.) Define the term ‘drug resistance’
Answer:
Drug resistance refers to the ability of microorganisms or cancer cells to survive and proliferate in the presence of drugs that would normally kill or inhibit them.
b.) Explain how multidrug resistance (MDR) develops in cancer cells and how it can be overcome
Answer:
Multidrug resistance (MDR) develops in cancer cells when they overexpress membrane transporters, such as P-glycoprotein, that can pump out drugs from the cell before they can exert their pharmacological effect. MDR can be overcome by using combination therapy with drugs that have different mechanisms of action or by co-administering drugs that can inhibit the efflux pump.
2.)
a.) Discuss the use of nanoparticles in targeted drug delivery
Answer:
Nanoparticles are submicron-sized particles that can be engineered to encapsulate drugs and target specific tissues or cells. Advantages of using nanoparticles in targeted drug delivery include improved drug solubility and stability, increased drug loading capacity, reduced toxicity and side effects, and enhanced cellular uptake and retention.
b.) Describe the pharmacological properties and therapeutic uses of cannabinoids
Answer:
The pharmacological properties of cannabinoids are mainly mediated by two receptors, CB1 and CB2, which are found in different tissues and cell types. Cannabinoids have been used therapeutically for pain relief, anti-inflammatory effects, anti-emetic effects, and as a treatment for epilepsy and multiple sclerosis. However, the use of cannabinoids is associated with several adverse effects, such as cognitive impairment, cardiovascular effects, and addiction potential.
3.)
a.)
i.) Define the term ‘drug’
Answer:
A drug is a chemical substance that alters the structure or function of the body when administered.
ii.) Explain how the potency of a drug is related to its efficacy
Answer:
The potency of a drug refers to the concentration required to achieve a particular effect, whereas efficacy refers to the maximum effect that can be achieved by the drug. Potency and efficacy are related in that a more potent drug will require a lower concentration to achieve the same effect as a less potent drug, but potency alone does not guarantee efficacy.
b.) Discuss the importance of chirality in drug design
Answer:
Chirality is the property of a molecule that is non-superimposable on its mirror image. Many drugs contain chiral centers and the different enantiomers can have different biological effects. In drug design, it is important to consider the stereochemistry of the molecule to optimize its pharmacological properties and minimize side effects.
4.)
a.)
i.) Define the term ‘bioavailability’
Answer:
Bioavailability refers to the fraction of an administered dose of a drug that reaches the systemic circulation unchanged and is available to exert its pharmacological effect.
ii.) Explain how pH partitioning affects the absorption of weakly acidic and weakly basic drugs in the body
Answer:
pH partitioning refers to the distribution of a drug between its ionized and non-ionized forms in different body compartments with different pH values. Weakly acidic drugs are predominantly non-ionized in acidic environments, such as the stomach, and are better absorbed there, while weakly basic drugs are predominantly non-ionized in basic environments, such as the small intestine, and are better absorbed there.
5.)
a.)
i.) Discuss the mechanism of action of statins as cholesterol-lowering drugs.
Answer:
Statins are cholesterol-lowering drugs that inhibit the enzyme HMG-CoA reductase, which is involved in the biosynthesis of cholesterol in the liver. By reducing the production of cholesterol, statins increase the expression of low-density lipoprotein (LDL) receptors on the surface of liver cells, leading to increased clearance of LDL from the blood and a decrease in LDL cholesterol levels.
ii.) Explain the role of enzyme inhibitors in the treatment of HIV infections
Answer:
Enzyme inhibitors are used in the treatment of HIV infections to inhibit the activity of the viral protease or reverse transcriptase enzymes, which are essential for viral replication.
iii.) Describe the mechanism of action of monoclonal antibodies as anticancer drugs.
Answer:
Monoclonal antibodies are laboratory-produced antibodies that can bind specifically to cancer cells and induce an immune response against them. They can target cancer cell surface markers or growth factors and inhibit cell proliferation, induce apoptosis, or activate the immune system.
6.)
a.) Penicillin is an antibiotic used to treat bacterial infections.
i.) Discuss the mechanism of action of penicillin in inhibiting bacterial cell wall synthesis.
Answer:
Penicillin inhibits bacterial cell wall synthesis by irreversibly binding to and inhibiting the enzyme transpeptidase, which is responsible for cross-linking peptidoglycan chains in the cell wall. This leads to weakened cell walls and eventually cell lysis.
ii.) Describe the main types of antibiotic resistance mechanisms and explain how they arise.
Answer:
The main types of antibiotic resistance mechanisms are: enzymatic degradation of the antibiotic, modification of the antibiotic target site, and efflux pumps that actively pump out the antibiotic from the cell. Antibiotic resistance arises through genetic mutations or acquisition of resistance genes from other bacteria through horizontal gene transfer.
7.)
a.) The following structures show four different classes of antidepressants:
1.) 2.)
2.)
3.) 4.)
4.)
i.) Name each class of antidepressants shown.
Answer:
Class 1: Selective Serotonin Reuptake Inhibitors (SSRIs)
Class 2: Monoamine Oxidase Inhibitors (MAOIs)
Class 3: Tricyclic Antidepressants (TCAs)
Class 4: Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs)
ii.) For each class of antidepressants, explain the mode of action and give an example of a drug in that class.
Answer:
Class 1: SSRIs work by selectively inhibiting the reuptake of serotonin, thereby increasing its concentration in the synaptic cleft. Example drug: fluoxetine (Prozac).
Class 2: MAOIs work by irreversibly inhibiting the enzyme monoamine oxidase, which is responsible for the breakdown of serotonin, norepinephrine, and dopamine. Example drug: phenelzine (Nardil).
Class 3: TCAs work by blocking the reuptake of both serotonin and norepinephrine. Example drug: amitriptyline (Elavil).
Class 4: SNRIs work by selectively inhibiting the reuptake of both serotonin and norepinephrine. Example drug: venlafaxine (Effexor).
References:
Our Expert Tutors!
- 12+ Years
Cat 1 – ESS and Cat 2 – Biology. Chief of the IB program. Mentored 320+ students across various curricula.
- 9+ Years
IBDP Cat 1 – Biology. Specializes in IBDP and A Levels Biology. 10+ years in Medicine with seasoned professionals.
- 9+ Years
IBDP Cat 1 – Business Management, IBDP Cat 1 – TOK. Taught over 130+ students across 4+ countries.
- 9+ Years
IBDP Cat 1 & 2 November 2019. Specializes in Global Politics. Many students scored 7s; mentors 200+ students in assessments.
- 10+ Years
IBDP Cat 2 – English, IBDP Cat 2 – TOK. Qualifications as IB Examiner & Supervisor. Taught over 120+ students.
- 18 + Years
IBDP Cat 1 – Chemistry, IBDP Cat 3 – IA Chemistry, IBDP Cat 1 – TOK. Helped 2 out of 3 students achieve a 7 in IB Chemistry.