chemical bonding and structure Notes
Check Out Our Results

OXFORD UNIVERSITY
(QS:3)

IMPERIAL COLLEGE
(QS:6)

CORNELL UNIVERSITY (QS:16)

45/45 (IBDP)
GEORGIA INSTITUTE

43/45 (IBDP)
KELLY SCHOOL
Ionic bonding and structure
Ionic bonding
- Ions are formed when one or more electrons are transferred from one atom to another.
- The positively charged protons, located within the nucleus of the atom, are not transferred during chemical reactions. Electrons, however, positioned outside the nucleus, are less tightly held and outer electrons, known as valence electrons, can be transferred when atoms react together.
- When this happens the atom is no longer neutral, but instead carries an electric charge and is called an ion.
- Elements that have a small number of electrons in their outer shells (Groups 1, 2, and 13) will lose those electrons and form positive ions called cations. These elements are the metals.
- Elements that have higher numbers of electrons in their outer shells (Groups 15, 16, and 17) will gain electrons and form negative ions called anions. These elements are the non-metals.
- The electrostatic attraction that exists between the electric charges of a cation (a positive ion) and an anion (a negative ion) is referred to as an ionic bond.
- Some ions are composed of more than one atom, each of which has lost or gained electrons, giving rise to a charge. These species are called polyatomic ions, and many of them are found in commonly occurring compounds.
- The oppositely charged ions resulting from electron transfer are attracted to each other and are held together by electrostatic forces.
These forces are known as an ionic bond, and ions held together in this way are known as ionic compounds.
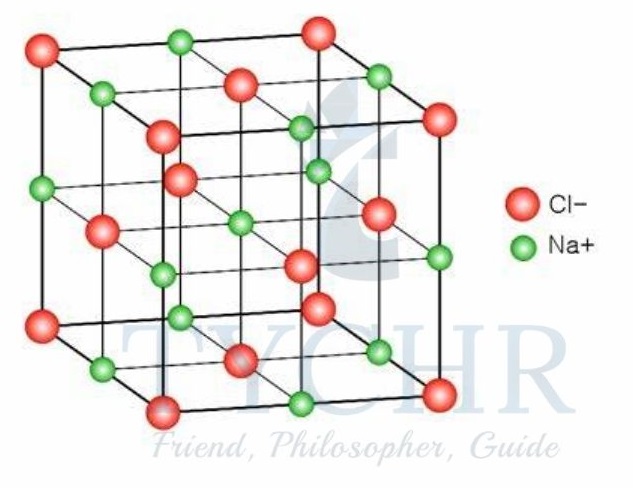
Ionic compounds have a lattice structure
- The forces of electrostatic attraction between ions in a compound cause the ions to surround themselves with ions of opposite charge.
- As a result, the ionic compound takes on a predictable three-dimensional crystalline structure known as an ionic lattice.
- The term coordination number is used to express the number of ions that surround a given ion in the lattice.
- For example, in the sodium chloride lattice, the coordination number is six because each Na+ ion is surrounded by six Cl– ions and each Cl– ion is surrounded by six Na+ ions.
The physical properties of ionic compounds
Melting points and boiling points
- Due to the strong electrostatic forces of attraction between the ions in their lattice structures, ionic compounds have high melting and boiling points.
- These compounds are therefore solids at room temperature, and will only melt at very high temperatures.
- Sodium chloride, for example, remains solid until about 800 °C.
- Volatility is a term used to describe the tendency of a substance to vaporize.
- Because of their strong electrostatic forces of attraction, ionic compounds have very low volatility.
Solubility
- Solubility refers to the ease with which a solid (the solute) becomes dispersed through a liquid (the solvent) to form a solution.
- The degree to which the solute’s separated particles are capable of forming bonds or attractive forces with the solvent is what determines its solubility.
Electrical conductivity
- The ability of a compound to conduct electricity depends on whether it contains ions that are able to move and carry a charge.
- Ionic compounds are not able to conduct electricity in the solid state as the ions are firmly held within the lattice and so cannot move.
- However, when the ionic compound is either present in the liquid state (molten), or dissolved in water (aqueous solution), the ions will be able to move.
- Therefore ionic compounds as liquids or aqueous solutions do show electrical conductivity.
Brittleness
- Ionic compounds are usually brittle, which means the crystal tends to shatter when force is applied.
- This is because the repelling forces cause the lattice to split as a result of ions of the same charge being displaced by movement within it.

Figure 2: Ionic and covalent compounds
Covalent bonding
Covalent bonding
- In ionic bonding we saw how atoms can either lose or gain electrons in order to attain a noble gas electron configuration.
- A second type of chemical bond exists, however, in which atoms share electrons with each other in order to attain a noble gas electron configuration. This type of bonding is covalent bonding, and it usually occurs between nonmetals.
- The shared pair of electrons is concentrated in the region between the two nuclei and is attracted to them both. It therefore holds the atoms together by electrostatic attraction and is known as a covalent bond.
- A molecule is made up of a group of atoms held together by covalent bonds.
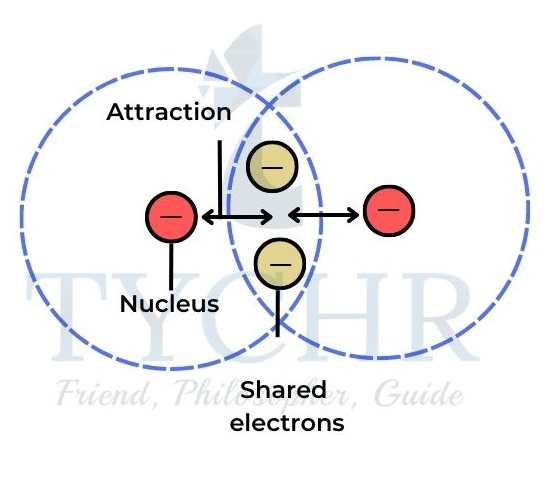
Figure 3: In a covalent bond the shared electrons are attracted to the nuclei of both atoms. - In a Lewis symbol representation, each element is surrounded by a number of dots (or crosses), which represent the valence electrons of the element.
- Fluorine is in group 17, so has seven valence electrons. Hence by acquiring one more electron, fluorine would attain a noble gas electron configuration with a complete octet of electrons.
- The Lewis symbol for fluorine is:
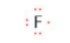
- A covalent bond is formed between two fluorine atoms when two fluorine atoms share one electron. Each fluorine atom then gains an additional electron to complete an octet of electrons.This covalent bond is a single bond and the shared pair can be represented by a line:
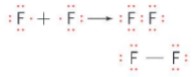
- Note that in this Lewis structure of F2 there are a total of six non-bonding pairs of electrons (often called lone pairs) and one bonding pair of electrons.
Bond strength and bond length
- We have molecules with single, double, and triple covalent bonds.
- Bond strength: The trend in bond strength is: ≡ > = > −
- This means that a triple bond is more powerful than a double bond, which in turn is more powerful than a single bond.
- Bond length: This is the opposite trend to bond strength: − > = > ≡
- A single bond has a shorter length than a double bond, which in turn has a shorter length than a triple bond.
Comparison of covalent bonds and ionic bonds
Properties | Covalent bonds | Ionic bonds |
Occurs between: | Two non-metals | One metal and one non-metal |
Melting and boiling point | Low | High |
Shape | Definite shape | No definite shape |
Polarity | Low | High |
Solubility | Insoluble in water | Soluble in water |
State at room temperature | Liquid or gaseous | Solid |
Conductivity | Do not conduct electricity. | Conduct electricity when molten or dissolved in water. |
Volatilities | May be volatile | Have low volatilities. |
Examples | Methane (CH4), Hydro Chloric acid (HCL). | Sodium Chloride (NaCl), Sulfuric Acid (H2SO4). |
Covalent structures
Lewis (electron dot) structures
- A Lewis symbol, which shows the number of valence electrons of an element represented by either dots or crosses.
- In such a representation it is important to distinguish between:
- Bonding pairs of electrons (showing the covalent bond as single, double, or triple bonds) and
- Non-bonding pairs of electrons, often called the lone pairs, which are pairs of electrons not involved in the bonding.
- The Lewis structures of carbon dioxide, CO2, and carbon monoxide, CO, which contain multiple bonds, can be represented as shown in figure 4.

Figure 4: Lewis structures of CO
Lewis (electron dot) structures of cations and anions and ionic compounds
- Lewis structures can be written not only for neutral molecules but also for cations and anions.
- In a compound containing both a cation and an anion there is an electrostatic attraction between the oppositely charged ions, which forms the ionic bond.
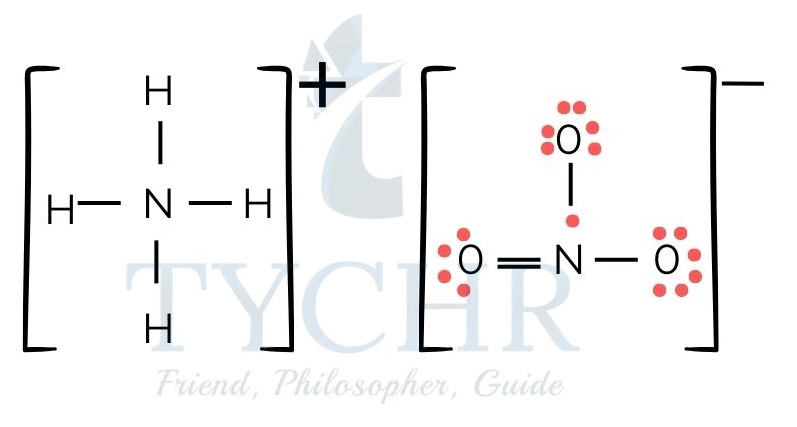
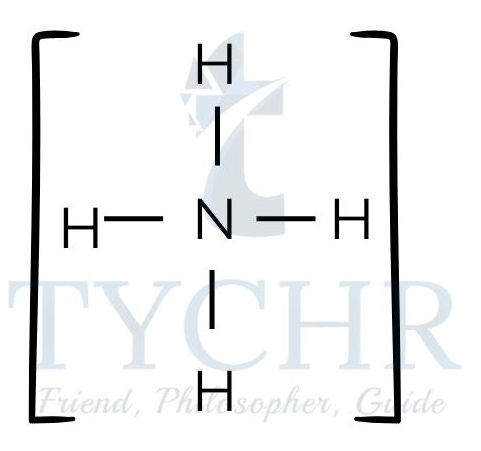
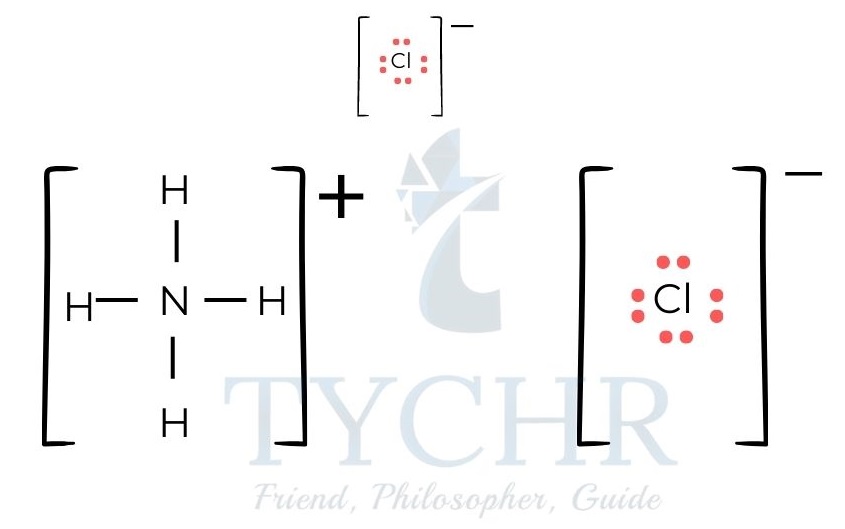
Figure 5: (a) Lewis structure of ammonium nitrate. (b) Lewis structure of ammonium chloride
Valence shell electron pair repulsion (VSEPR) theory
- The theory of valence shell electron pair repulsion (VSEPR) can be used to figure out how covalent molecules look.
- The repulsion applies to electron domains, which can be single, double, or triple bonding electron pairs, or non-bonding pairs of electrons.
- The total number of electron domains around the central atom determines the geometrical arrangement of the electron domains.
- The shape of the molecule is determined by the angles between the bonded atoms.
- Non-bonding pairs (lone pairs) have a higher concentration of charge than a bonding pair because they are not shared between two atoms, and so cause slightly more repulsive than bonding pairs.
- The repulsion decreases in the following order: lone pair–lone pair > lone pair–bonding pair > bonding pair–bonding pair.
Species with two electron domains
- Molecules with two electron domains will position them at 180° to each other, giving a linear shape to the molecule.
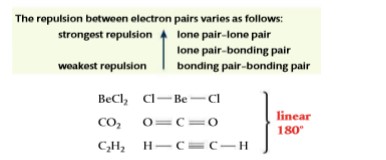
Figure 6: linear geometry
Species with three electron domains
- Molecules with three electron domains will position them at 120° to each other, giving a triangular planar shape to the electron domain geometry. If all three electron domains are bonding, the shape of the molecule will also be triangular planar.
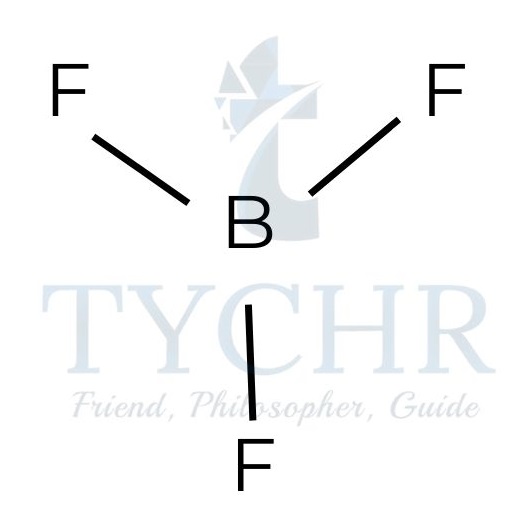
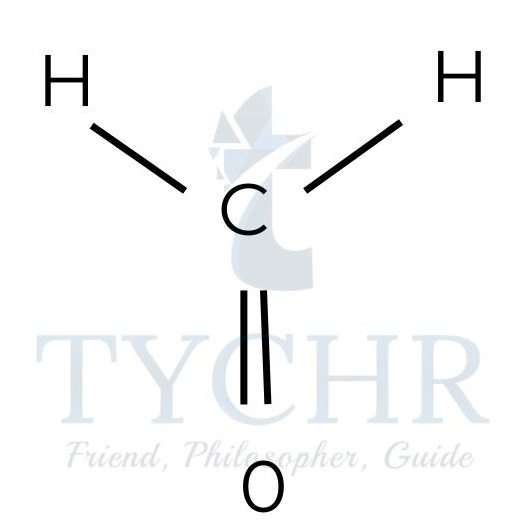
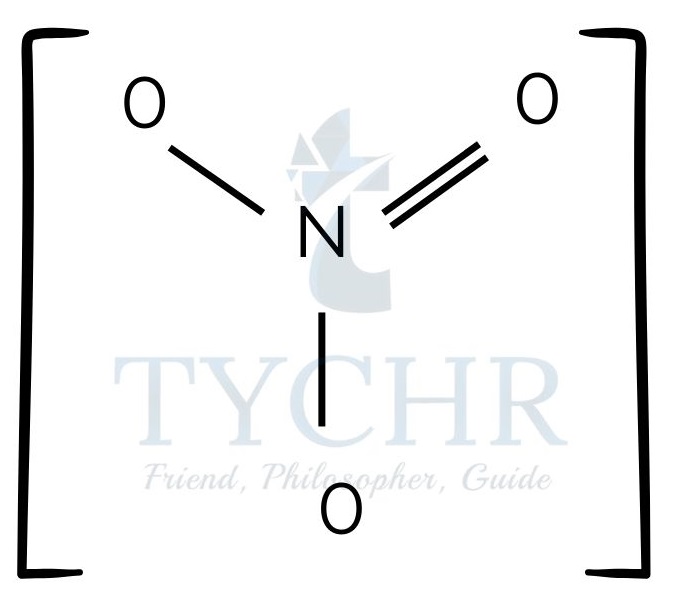
Figure 7: Triangular planar
Species with four electron domains
- Molecules with four electron domains will position them at 109.5° to each other, giving a tetrahedral shape to the electron domains. If all four electron domains are bonding, the shape of the molecule will also be tetrahedral.

Figure 8: Tetrahedral shape
Resonance structure
- In resonance, a particular molecule or ion is represented by two or more Lewis structures.
- For a molecule or ion, a resonance structure is one of two or more alternative Lewis structures that cannot be fully described by one Lewis structure alone.
- Instead of being localized between a pair of atoms, electrons in delocalization are shared by more than two atoms in a molecule or ion.
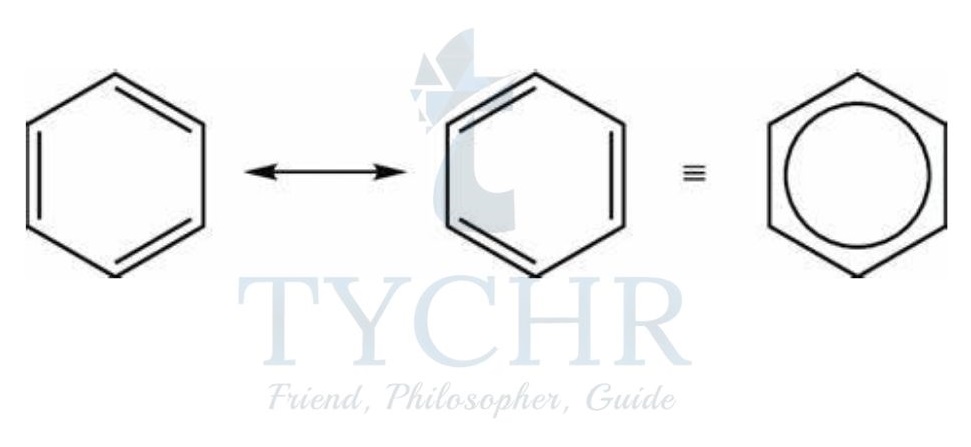
Figure 9: (a) Two Kekulé structures of benzene showing resonance. (b) Representation of benzene showing the delocalized nature of its π electrons
Allotropes
- Allotropes of the same element can vary in both physical and chemical properties.
- Carbon is one of the most fascinating elements in the periodic table, and life forms on Earth are based on carbon.
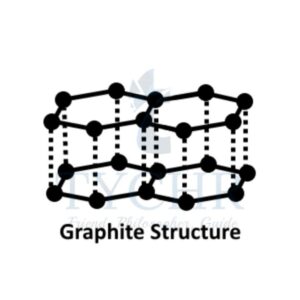
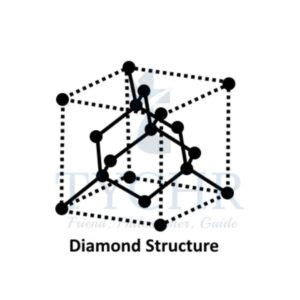
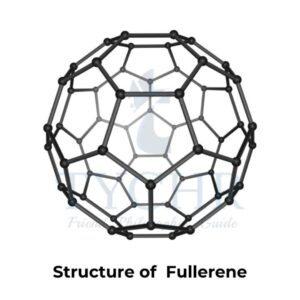
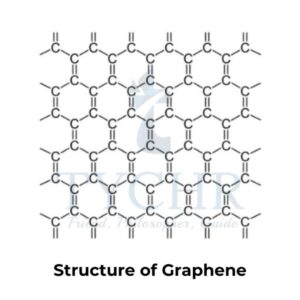
- Carbon has a number of allotropes: graphite, diamond, graphene, and C60 fullerene.
- Graphite, diamond, and graphene are examples of covalent network solids.
- A solid with a covalent network is one in which the atoms are held together by covalent bonds in a massive three-dimensional lattice structure (in chains or large networks).
- Another well known example of a covalent network solid is quartz, which is silicon dioxide, SiO2.
- In contrast, C60 fullerene is molecular.
Difference between Graphite, Diamond, Fullerene C60 and Graphene
Properties | Graphite | Diamond | Fullerene C60 | Graphene |
Structure |
Each C atom is sp2 hybridized and covalently bonded to 3 others, forming hexagons in parallel layers with bond angles of 120°. The layers are held only by weak London dispersion forces so they can slide over each other. |
Each C atom is sp3 hybridized and covalently bonded to 4 others tetrahedrally arranged in a regular repetitive pattern with bond angles of 109.5°. |
In a 60-carbon sphere with 12 pentagons and 20 hexagons, each C atom is sp2 hybridized and bonded. Structure is a closed spherical cage in which each carbon is bonded to 3 others. |
Similar to graphite, three C atoms are covalently bonded to each other, forming 120°-bonding hexagons. However, due to its single layer, it only exists as a two-dimensional material. It is frequently referred to as a chicken wire or honeycomb structure. |
Electrical conductivity | good conductor of electricity; contains one delocalized, non-bonded electron per atom, giving it mobility. | unable to conduct electricity; Because they are all bonded, electrons cannot move. | a semiconductor with some electron mobility at normal temperature and pressure; accepts electrons with ease, resulting in negative ions. | Very good conductor of electricity; Mobility of electrons across layers is provided by one delocalized electron per atom. |
Thermal conductivity | Unless the heat can be forced to flow parallel to the crystal layers, it is not a good conductor. | Thermally conducts heat more effectively than metals. | Very little ability to conduct heat. | The highest level of known thermal conductivity, surpassing diamond. |
Appearance | Non-lustrous, grey crystalline solid. | Highly transparent, lustrous crystal. | Yellow crystalline solid, soluble in benzene. | Almost completely transparent. |
Special properties | Soft and slippery as a result of layers sliding over one another; brittle; extremely low melting point; carbon’s most stable allotrope | the natural substance known to be hardest; It is unbreakable by anything; brittle; extremely low melting point. | Strong and lightweight; reacts with K to produce crystalline material that is superconducting; low point of melting | The smallest material to ever exist, with a thickness of just one atom; but they are also the strongest, 100 times more powerful than steel; very adaptable; extremely low melting point. |
Uses | a dry grease; using pens; in electrolysis, electrode rods. | polished for use in ornaments and jewellery; tools and equipment for cutting and grinding glass | medical and industrial instruments, lubricants, and devices for binding specific target molecules; Nanotubes, nanobuds, and catalysts made from related forms are utilized in the electronics industry as capacitors. | Grids, photovoltaic cells, touch screens, and high-performance electronic devices are all examples of TEM (transmission electron microscopy) grids; Numerous applications remain in development. |
Silicon Dioxide, SiO2 (quartz)
- Silicon dioxide, SiO2, often called silica, is found in its amorphous form (that is, a solid with no ordered structure) as sand. In its most common crystalline form it is called quartz.
- Quartz also forms a giant covalent structure based on a tetrahedral arrangement.
- But here the bonds are between silicon and oxygen atoms, where each Si atom is covalently bonded to four O atoms, and each O to two Si atoms. You can think of the oxygen atoms as forming bridges between the tetrahedrally bonded silicon atoms.
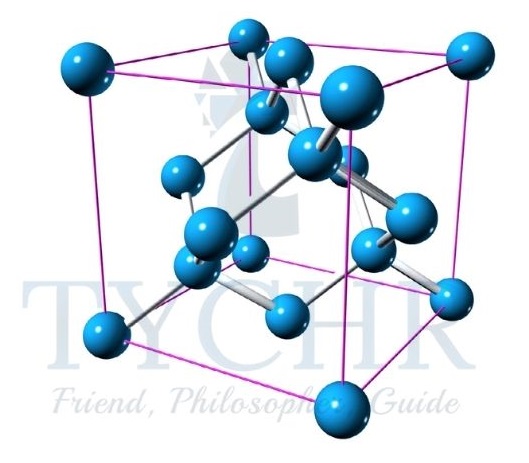
Figure 10: The silicon crystal structure and the arrangement of bonds around a central silicon atom. 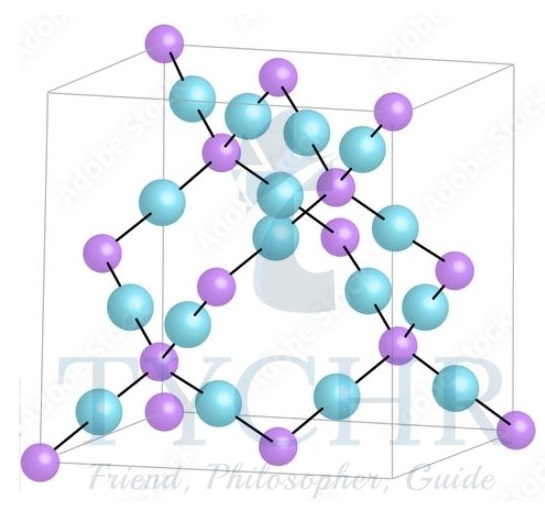
Figure 11: The structure of quartz SiO2. - As the atoms are strongly held in tetrahedral positions that involve all four silicon valence electrons, the structure has the following properties:
- strong;
- insoluble in water;
- high melting point;
- Non-conductor of electricity.
Intermolecular forces
London (dispersion) forces
- Because the shared electrons are pulled equally by the two chlorine atoms, non-polar molecules like chlorine, Cl2, do not have a permanent charge separation within their bonds.
- To put it another way, they lack a dipole that is always in use.
- However, because electrons behave somewhat like mobile clouds of negative charge, the density of this cloud may at any one moment be greater over one region of a molecule or atom than over another region.
- When this occurs, a weak dipole known as a temporary or instantaneous dipole is formed.
- This will not last for more than an instant as the electron density is constantly changing, but it may influence the electron distribution in a neighbouring atom or molecule, causing an induced dipole.
- As a result, weak forces of attraction, known as London (dispersion) forces, will occur between opposite ends of these two temporary dipoles in the molecules.
- These are the weakest form of intermolecular force.
- Their strength increases with increasing molecular size; this is because the greater number of electrons within a molecule increases the probability of temporary dipoles developing.
- Non-polar molecules are only held together by London forces, or dispersion forces.
- Because it takes relatively little energy to break the weak London (dispersion) forces and separate the molecules from one another, such molecules typically have low melting and boiling points.
.
Figure 12: Instantaneous dipole–induced dipole cause London (dispersion) forces between molecules of Cl2.
Dipole–dipole attraction
- Due to the difference in electronegativity between the bonded atoms, polar molecules like hydrogen chloride, HCl, have a charge separation that lasts forever within their bonds.
- The molecule has an electron-deficient end with a partial positive charge (δ+) and an electron-rich end with a partial negative charge (δ–).
- The term for this is a permanent dipole.
- A force known as a dipole–dipole attraction is created when molecules with opposite charges attract one another.
Figure 13: Permanent dipoles cause forces of dipole– dipole attraction between molecules of HCl.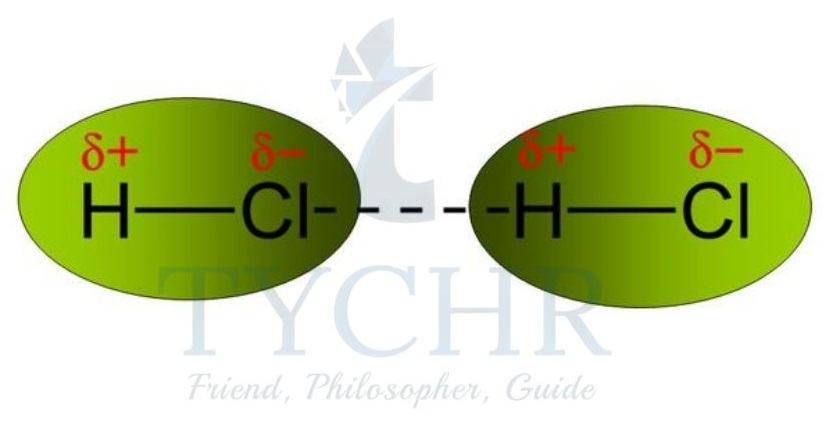
Figure 13: Permanent dipoles cause forces of dipole– dipole attraction between molecules of HCl. - However, due to their origin in permanent rather than instantaneous dipoles, dipole–dipole forces are typically stronger than London dispersion forces.
- Because of these forces, polar compounds have higher melting and boiling points than non-polar compounds of the same molecular mass.
- London dispersion forces and dipole–dipole attractions are both included under the umbrella term “van der Waals’ forces.”
- It also covers dipole–induced dipole, a less common type of attraction.
- In other words, all forces between molecules that do not involve bond formation or electrostatic attraction between ions are referred to as van der Waals’ forces.
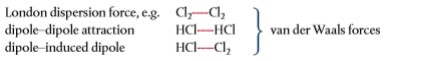
Hydrogen bonding
- A particularly powerful kind of intermolecular force known as a hydrogen bond occurs when hydrogen is covalently bonded to a very electronegative atom (such as oxygen, nitrogen, or fluorine).
- The hydrogen bond is in essence a particular case of dipole–dipole attraction.
- The large electronegativity difference between hydrogen and the bonded fluorine, oxygen, or nitrogen results in the electron pair being pulled away from the hydrogen.
- The hydrogen now exerts a strong attractive force on a lone pair in the electronegative atom of a neighbouring molecule due to its small size and lack of other electrons to shield the nucleus. The hydrogen bond is this.
- Intermolecular attraction is strongest in the form of hydrogen bonds.
- As a result, the boiling points of the substances that contain them rise significantly above what would be expected given their molar mass.
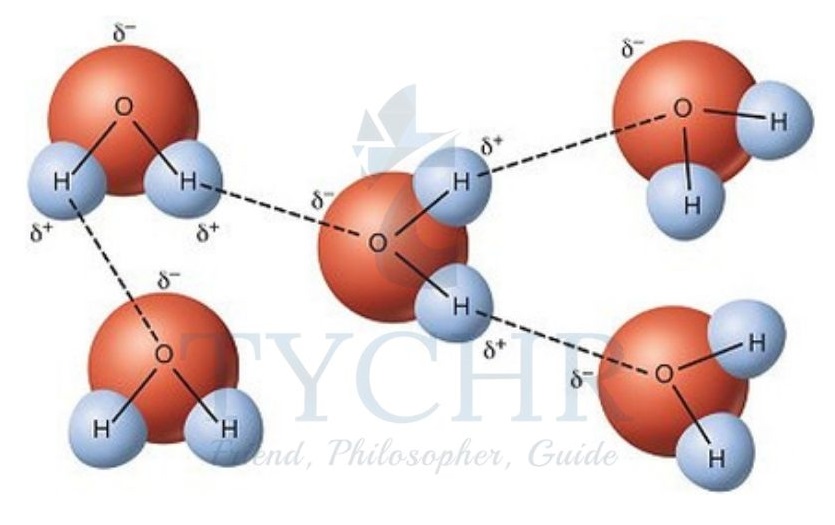
Figure 14: Hydrogen bonding between water molecules.
Summary
Non-polar molecules | Polar molecules | London dispersion forcesMolecules which contain H– O, H–N, or H–F | |
London dispersion forces | ✔ | ✔ | ✔ |
Dipole–dipole attractions | ✔ | ✔ | |
Hydrogen bonding | ✔ |
Summary of physical properties
Ionic compounds | Polar covalent compounds | Non-polar covalent compounds | Giant covalent | |
Volatility | low | higher | highest | low |
Solubility in polar solvent, e.g. water | soluble | solubility increases as polarity increases | non-soluble | non-soluble |
Solubility in nonpolar solvent, | non-soluble | solubility increases as polarity | soluble | non-soluble |
Electrical conductivity | conduct when molten (l) or dissolved in water (aq) | non-conductors | non-conductors | non-conductors except graphite, graphene and semiconductivity of Si and fullerene |
Metallic bonding
Metallic bonding
- In the elemental state, when there is no other element present to accept the electrons and form an ionic compound, the outer electrons are held only loosely by the metal atom’s nucleus and so tend to ‘wander off’ or, more correctly, become delocalized.
- Delocalized electrons are not fixed in one position.
- This means that in metals they are no longer associated closely with any one metal nucleus but instead can spread themselves through the metal structure.
- The metal atoms without these electrons have become positively charged ions and form a regular lattice structure through which these electrons can move freely.
- In metals there is a force of electrostatic attraction between the lattice of cations and the delocalized electrons, and this is known as metallic bonding.
- The strength of the metallic bond is determined by:
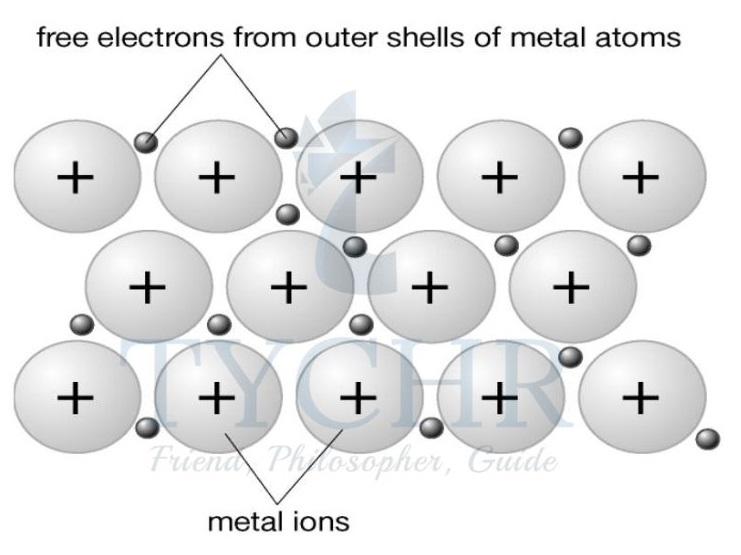
Figure 15: A model of metallic bonding. It is often described as a lattice of cations surrounded by a sea of electrons - the number of delocalized electrons;
- the charge on the cation;
- the radius of the cation.
- This model of metallic bonding provides an explanation for the physical properties that metals share. Below is a summary of them.
Metallic property | Explanation | Application |
good electrical conductivity | delocalized electrons are highly mobile, and so can move through the metal structure in response to an applied voltage | electrical circuits use copper |
good thermal conductivity | delocalized electrons and close packed ions enable efficient transfer of heat energy | cooking utensils |
malleable, can be shaped under pressure | The metallic bond remains intact while the conformation changes when pressure is applied because the movement of delocalized electrons through the cation lattice is essentially random and non-directional. | moulded into many forms including machinery and structural components of buildings and vehicles |
ductile, can be drawn out into threads |
| electric wires and cables |
high melting points | a lot of energy is required to break the strong metallic bonds and separate the atoms | high speed tools and turbine engines; tungsten has the highest melting point |
shiny, lustrous appearance | delocalized electrons in metal crystal structure reflect light | ornamental structures |
Alloys
- An alloy is a mixture that consists either of two or more metals, or of a metal (or metals) combined with an alloying element composed of one or more non-metals (for example, cast iron consists of the metal iron and the non-metal carbon).
- Solid alloys are held together by metallic bonding and typically consist of multiple metals. Alloys have enhanced properties, such as strength, hardness, and durability which differ from those of the parent metallic elements.
Name of alloy | Component metals | Properties and uses |
steel | iron with carbon and other elements | high tensile strength but corrodes, used as structural material |
stainless steel | iron with other elements such as nickel and chromium | widely used in domestic and industrial appliances due to strength and corrosion resistance |
brass | copper and zinc | variety of plumbing fittings |
bronze | copper and tin | coins, medals, tools, heavy gears |
pewter | tin and antimony and copper | decorative objects |
duralumin | aluminium, copper, and manganese | aircraft, boats, and machinery due to high strength and resistance to corrosion |
Nichrome | nickel and copper | heating elements in toasters, electric heaters |
solder | lead and tin | low melting point, used in joining two metals together, especially in electric circuitry |
sterling silver | silver and copper | jewellery, art objects |
Further aspects of covalent bonding and structure
Formal charge
- A Lewis (electron dot) structure is a convenient way of showing how the valence electrons are distributed in a covalent molecular species or a polyatomic ion.
- Sometimes a number of different Lewis structures can be drawn that all obey the octet rule.
- A useful approach in deciding which Lewis structure is the most appropriate is to determine the formal charge (FC) of the atoms present in the molecule or ion.
FC = (number of valence electrons) – 1/2 (number of bonding electrons) – (number of non- bonding electrons) - In the molecule tetrachloromethane, CCl4 (figure 1) the formal charge on each atom is calculated as follows: FC(C) = (4) -1/2(8)- 0 = 0 ; FC(Cl) = (7) -1/2- (2)-6 = 0.
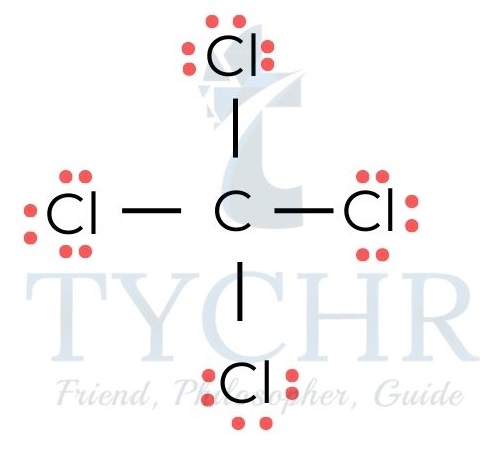
Figure 16: Lewis structure of tetrachloromethane, CCl4 - If there are a number of possible Lewis structures that all obey the octet rule, the most reasonable one will be:
- the one with FC difference (ΔFC = FCmax- FCmin) closest to 0, and
- the one that has the negative charges located on the most electronegative atoms.
LP|LP > LP|BP > BP|BP
(LP = lone pair; BP = bonding pair)
Table 6: Summary of shapes of molecules predicted from VSEPR theory.
Number of electron domains | Electron domain geometry | Number of bonding electron domains | Number of nonbonding pairs of electrons | Molecular geometry | Example |
2 | linear | 2 | 0 | linear |  |
3 | planar triangular | 3 | 0 | planar triangular | 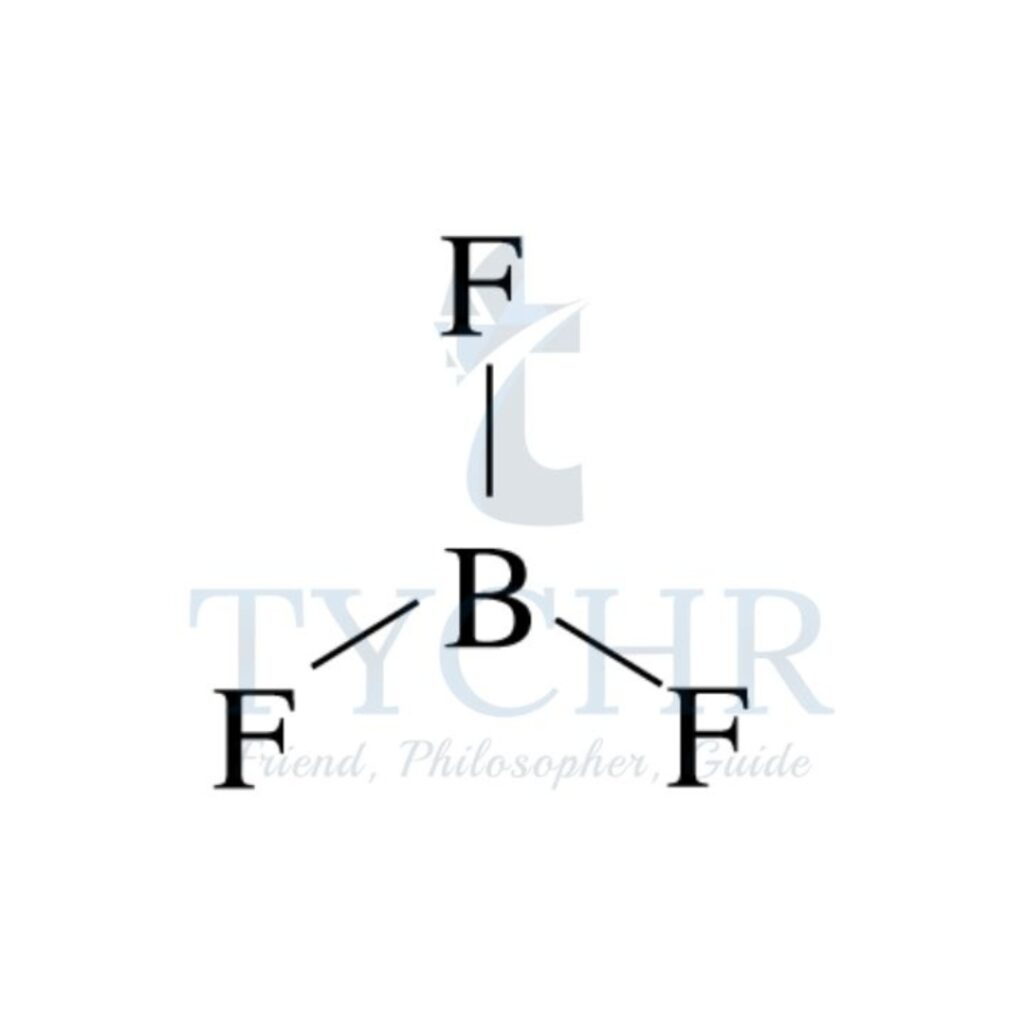 |
3 | planar triangular | 2 | 1 | V-shaped |  |
4 | tetrahedral | 4 | 0 | tetrahedral | 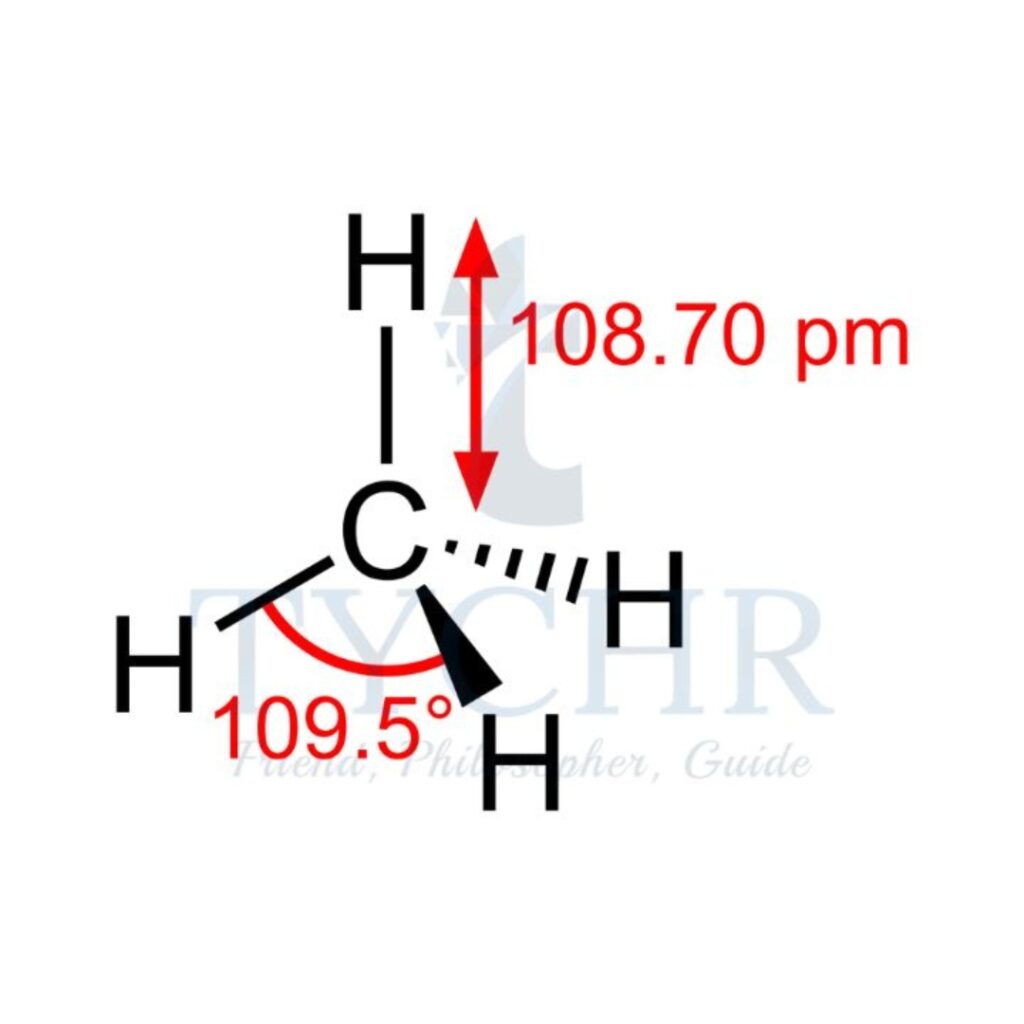 |
4 | tetrahedral | 3 | 1 | pyramidal | 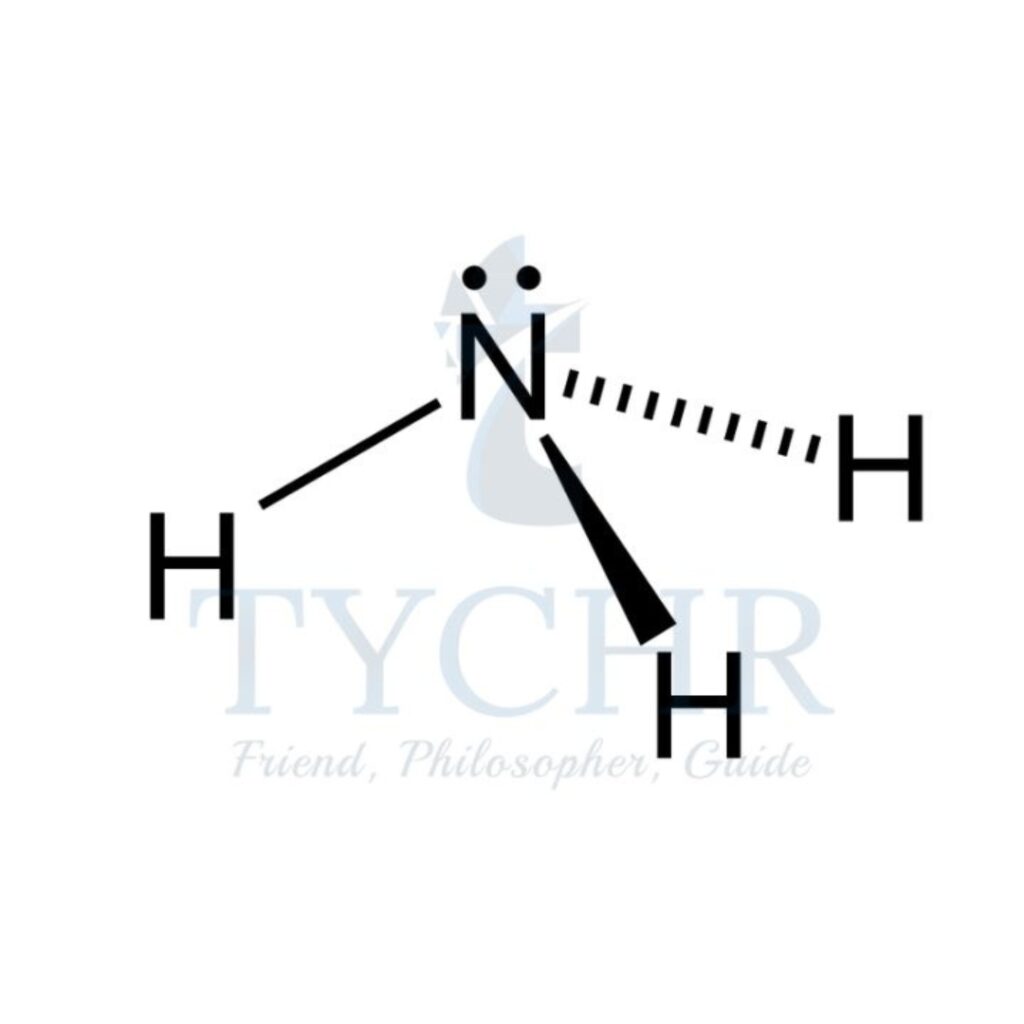 |
4 | tetrahedral | 2 | 2 | V-shaped | 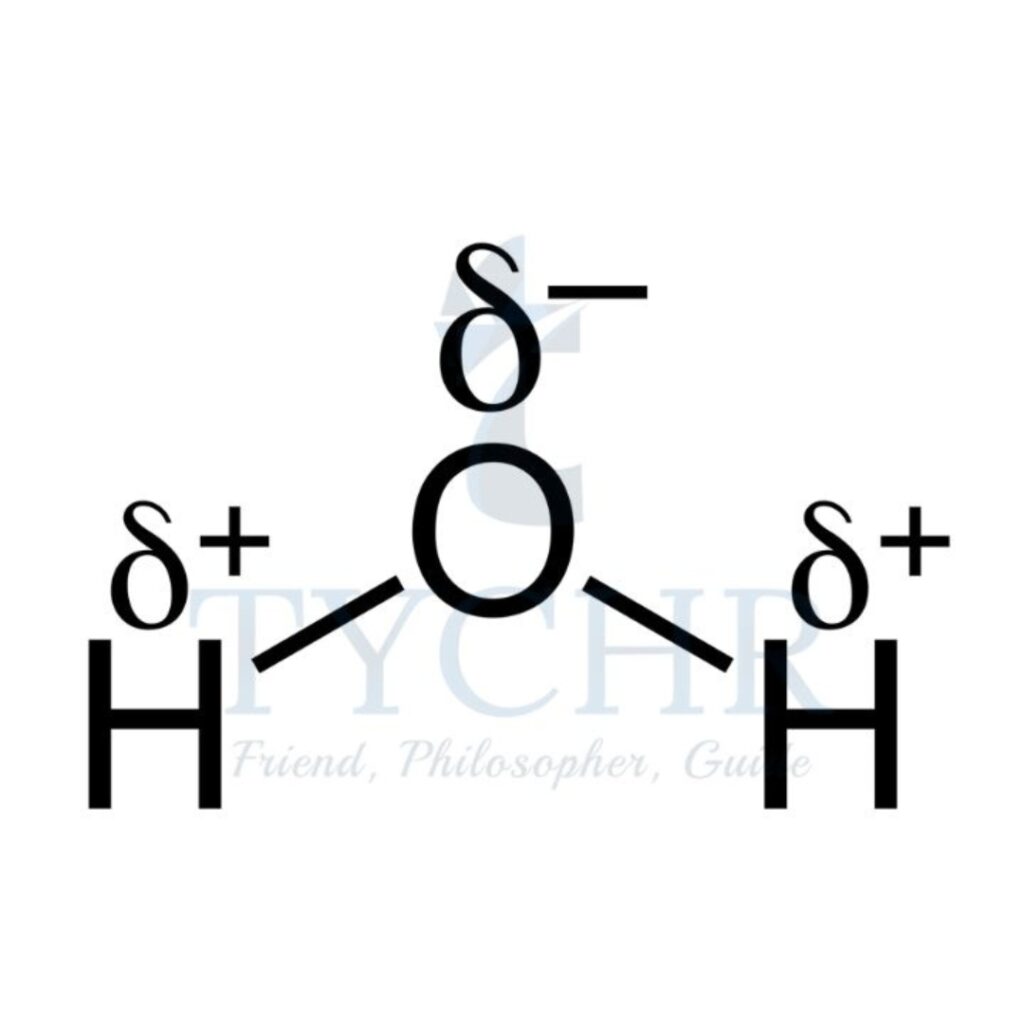 |
5 | triangular bipyramidal | 5 | 0 | triangular bipyramidal | 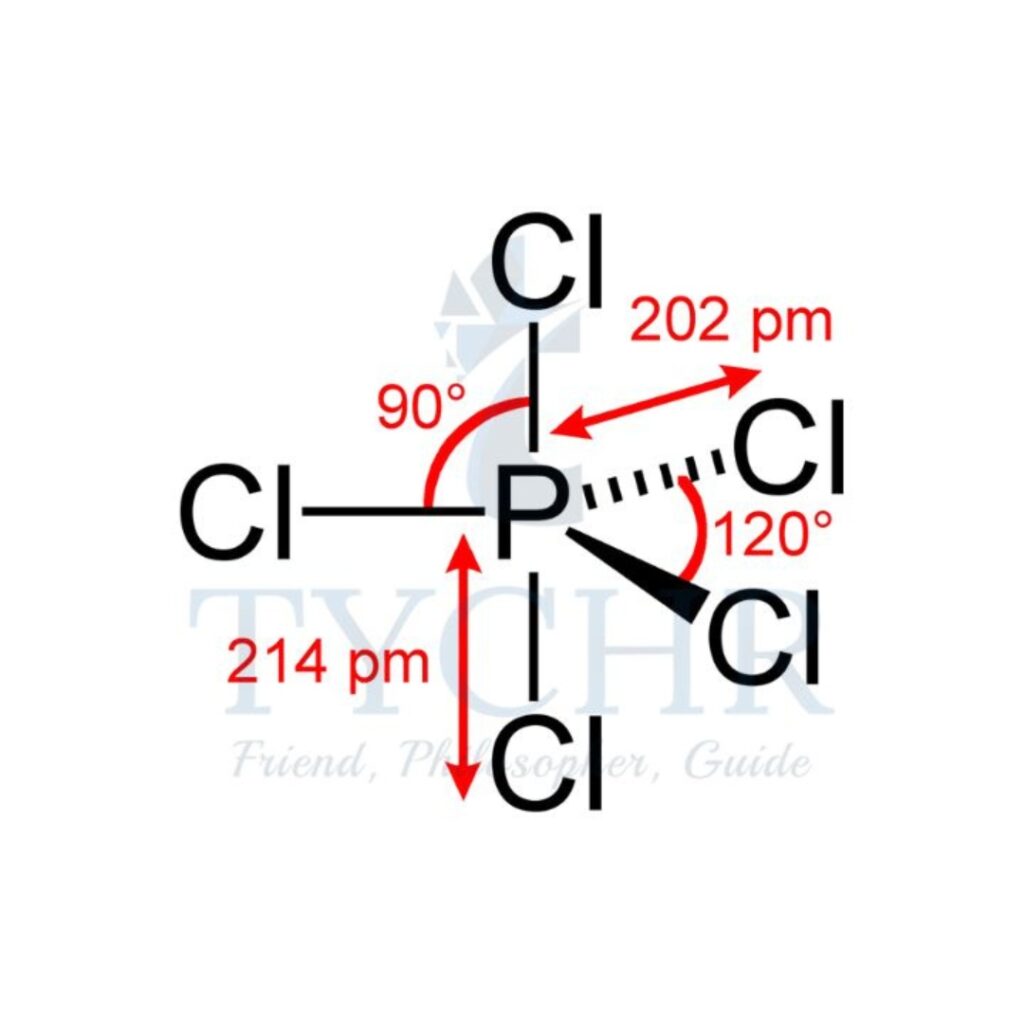 |
5 | triangular bipyramidal | 4 | 1 | unsymmetrical tetrahedron/see- saw | 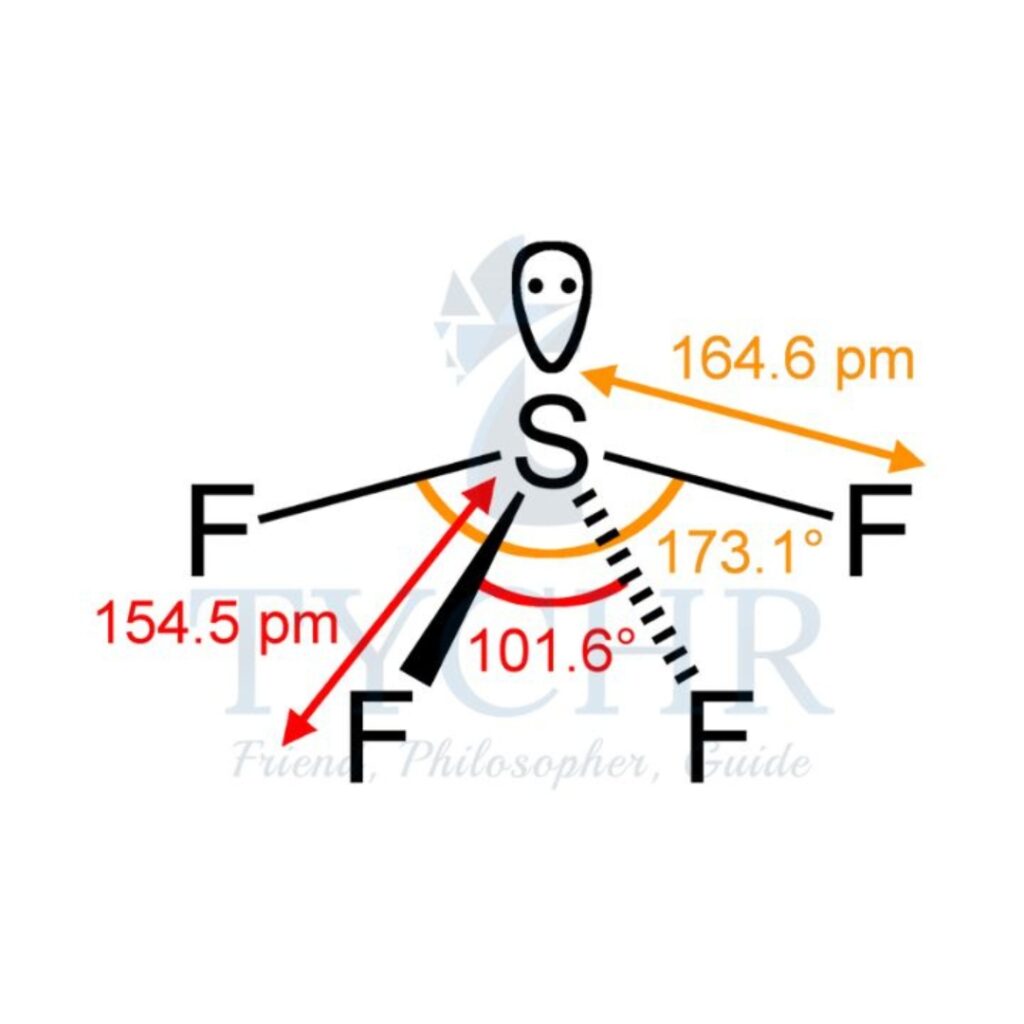 |
5 | triangular bipyramidal | 3 | 2 | T-shaped | 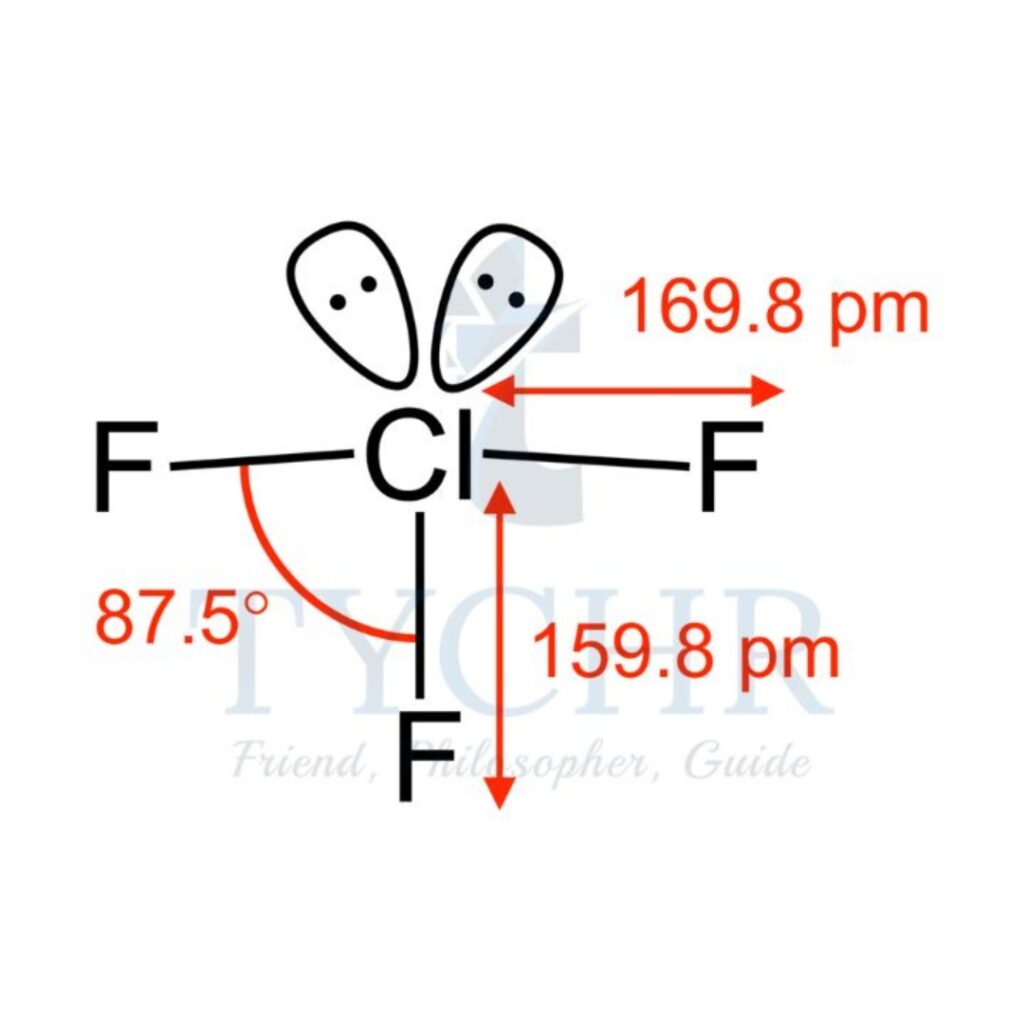 |
5 | triangular bipyramidal | 2 | 3 | linear | 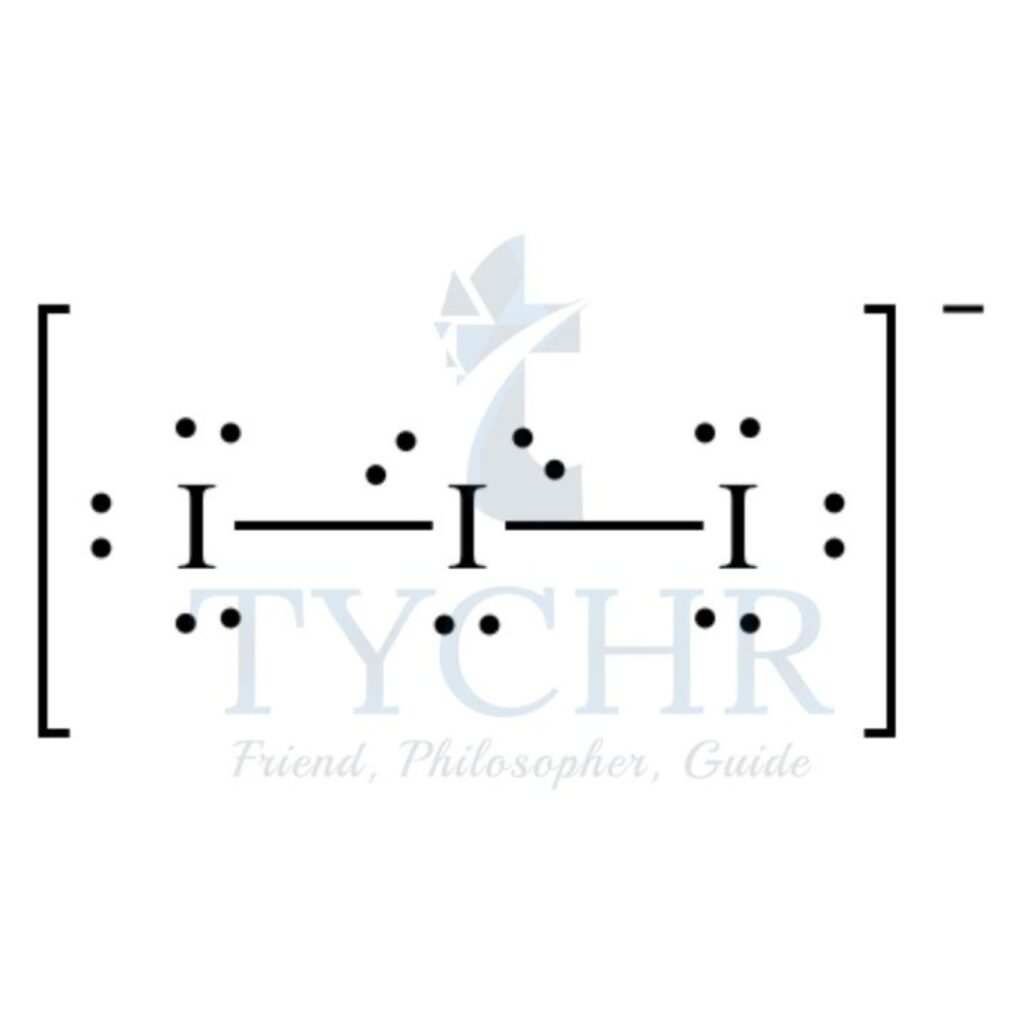 |
6 | octahedral | 6 | 0 | octahedral |  |
6 | octahedral | 5 | 1 | square pyramidal | 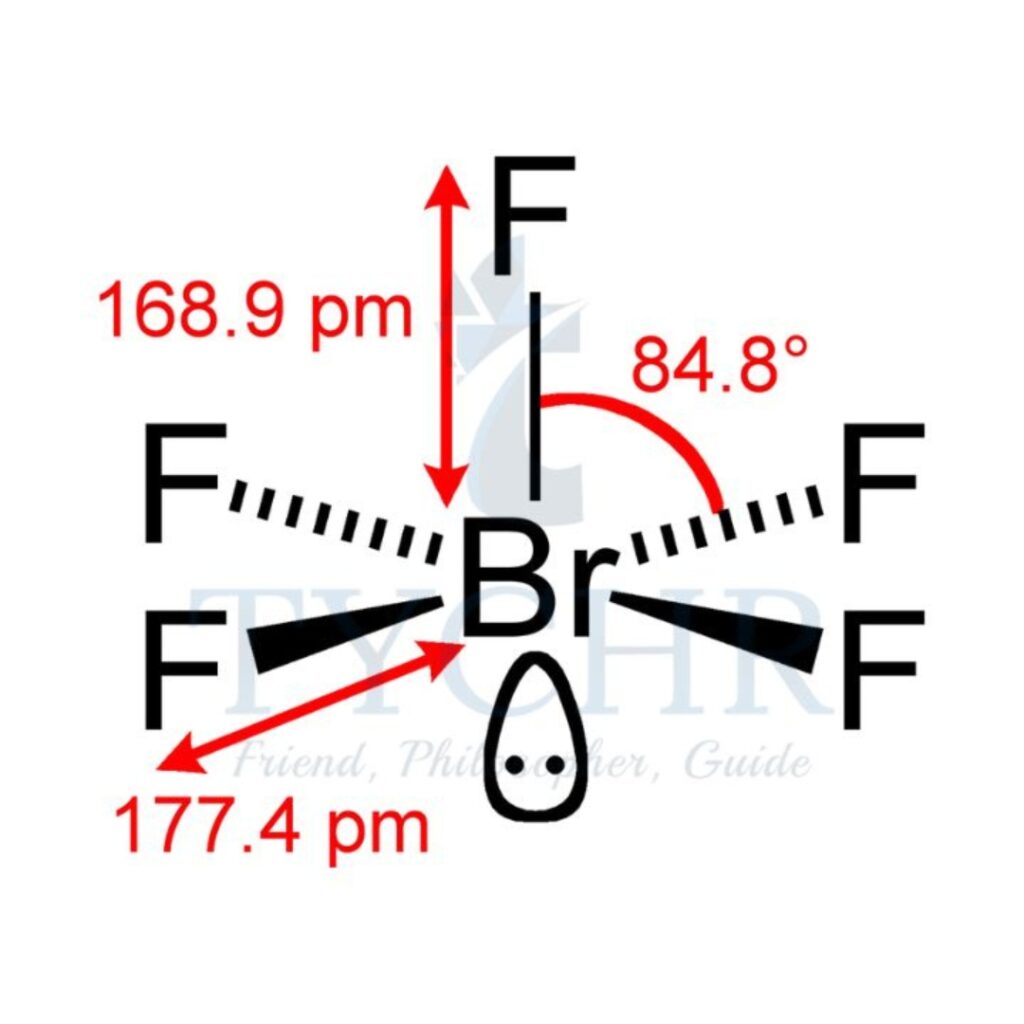 |
6 | octahedral | 4 | 2 | square planar | 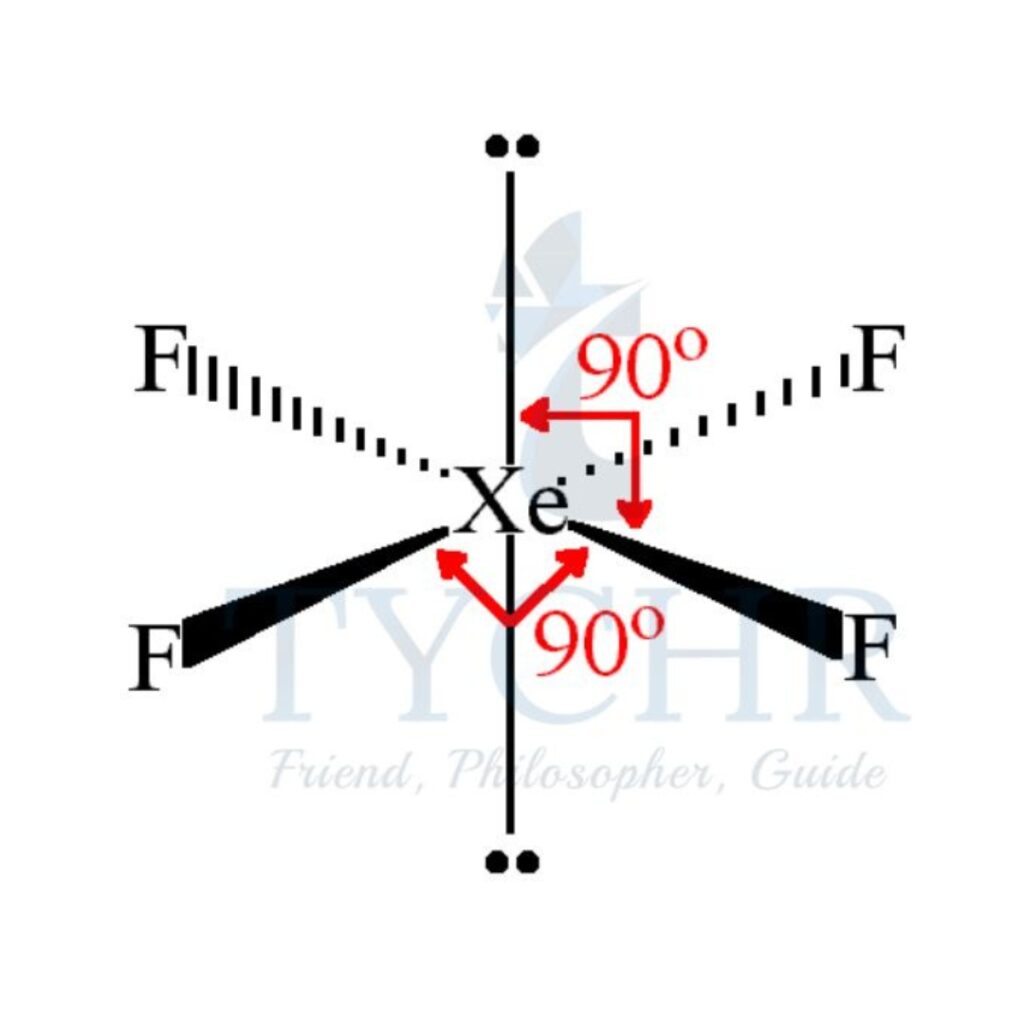 |
Overlap of atomic orbitals: Sigma (σ) and pi (π) bonding
- A single covalent bond consists of two electrons shared between two atoms A and B.
- The single bond, represented by a stick, is a sigma bond (σ).
- A double covalent bond consists of four electrons, two pairs, shared between two atoms A and B.
- The double bond, represented by two sticks, is a sigma plus pi bond (σ + π).
- A triple covalent bond consists of six electrons, or three pairs, shared between two atoms A and B.
- The triple bond, represented by three sticks, is a sigma plus 2 two pi bonds (σ + 2π).
Figure 16: Covalent bonds - For atomic orbitals to overlap and form molecular orbitals they must be relatively close in energy, and the symmetry of the atomic orbitals must be identical. X atomic orbitals combine to form x new molecular orbitals. There are three possible outcomes:
1) bonding orbital: sigma (σ) or pi (π) orbital
2) anti-bonding orbital: sigma star (σ*) or pi star (π*) orbital
3) non-bonding situation.
Ozone is an essential component of the stratosphere
- The layer of gas tens of kilometres above us, known as the atmosphere, is essential for life on Earth.
- It prevents some of the harmful radiation from the Sun from reaching Earth by trapping heat and maintaining a temperature suitable for life.
- The region from approximately 12–50 kilometres above the Earth is known as the
stratosphere.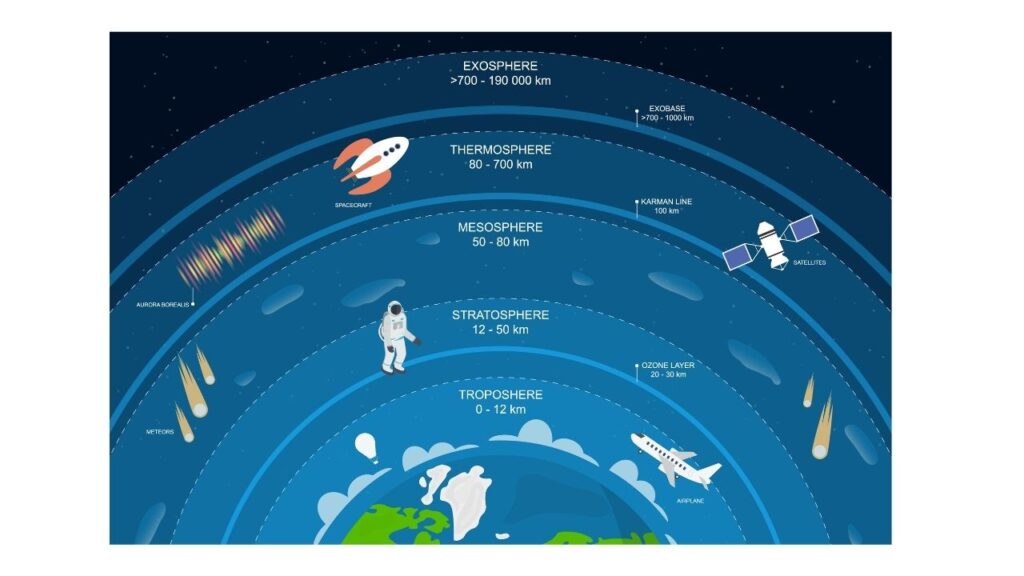
Figure 17: Regions of the atmosphere. The ozone layer is in the lower part of the stratosphere, approximately 20–30 kilometres above Earth. - The lower part of the stratosphere, known as the ozone layer, contains 90% of the atmospheric ozone, although the concentration is less than 10 parts per million.
- Ozone levels are maintained through a cycle of reactions involving the formation and breakdown of oxygen and ozone.
- The symbol O· represents a species known as a free radical that has an unpaired electron and so is highly reactive.
Catalytic destruction of ozone
- Ozone’s ability to absorb UV radiation also means that it is unstable, and it reacts easily with compounds found in the stratosphere that have been released through human activities.
- Chief amongst these are nitrogen oxides, known as NOx, and chlorofluorocarbons, known as
CFCs. - These compounds produce highly reactive free radicals that catalyse the decomposition of ozone to oxygen.
- The reactions of nitrogen oxides with ozone are as follows:
NO·(g) + O3(g) → NO2·(g) + O2(g)
NO2·(g) + O·(g) → NO·(g) + O2(g) - NO·(g) has acted as a catalyst because it is regenerated during the reaction and the net change is the breakdown of ozone:
O3(g) + O·(g) → 2O2(g)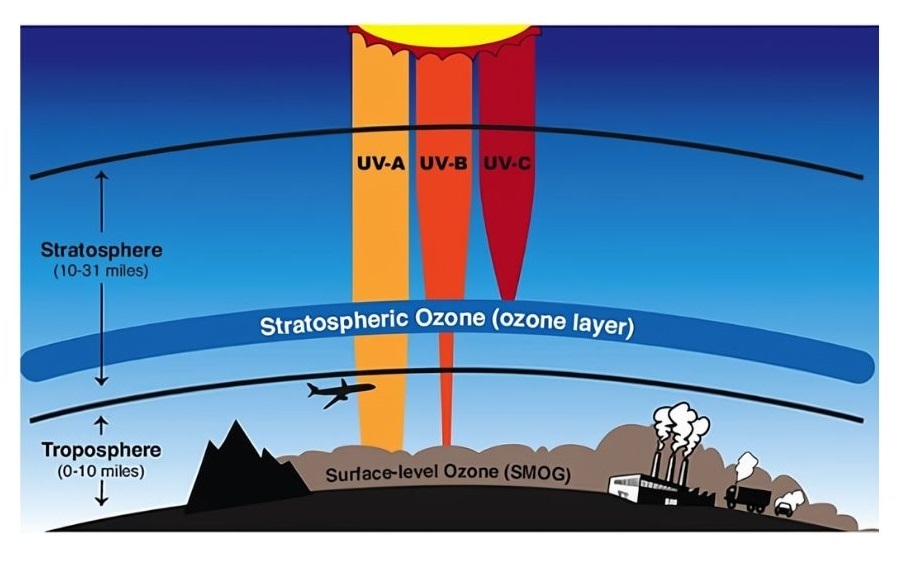
Figure 18: The ozone layer is responsible for absorbing much of the harmful UV radiation.
Hybridization
Hybridization
- On the same atom, different kinds of atomic orbitals are combined to form a hybrid orbital.
- Unequal atomic orbitals within an atom mix to form new hybrid atomic orbitals which are the same as each other, but different from the original orbitals.
- This mixing of orbitals is known as hybridization.
- Hybrid orbitals have different energies, shapes, and orientation in space from their parent orbitals and are able to form stronger bonds by allowing for greater overlap.
- Hybridization can involve different numbers and types of orbital, leading to the formation of distinct hybrid orbitals. We will discuss those involving s and p orbitals here.
sp3 hybridization
- When carbon forms four single bonds, it undergoes sp3 hybridization, producing four equal orbitals.
- These orbitals orientate themselves at 109.5°, forming a tetrahedron. Each hybrid orbital overlaps with the atomic orbital of another atom forming four sigma bonds. For example, methane, CH4.
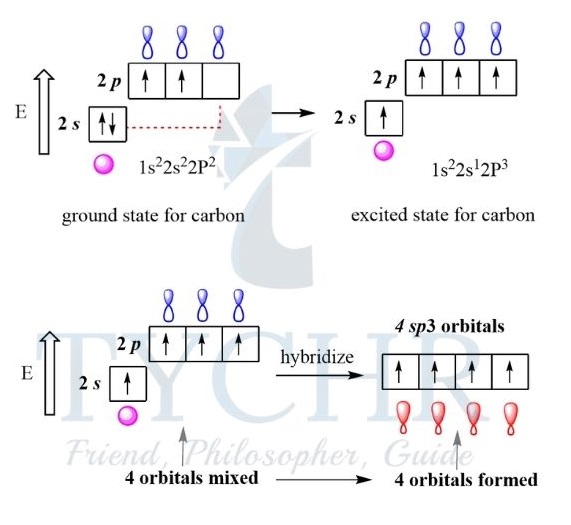
Figure 19: sp3 hybridization.
sp2 hybridization
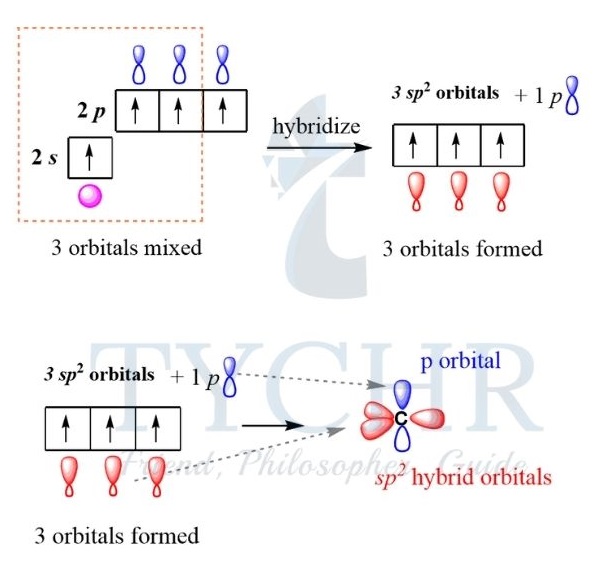
- When carbon forms a double bond, it undergoes sp2 hybridization, producing three equal orbitals.
- These orbitals orientate themselves at 120°, forming a triangular planar shape. Each hybrid orbital overlaps with a neighbouring atomic orbital, forming three sigma bonds. For example, ethene, C2H4.
sp hybridization
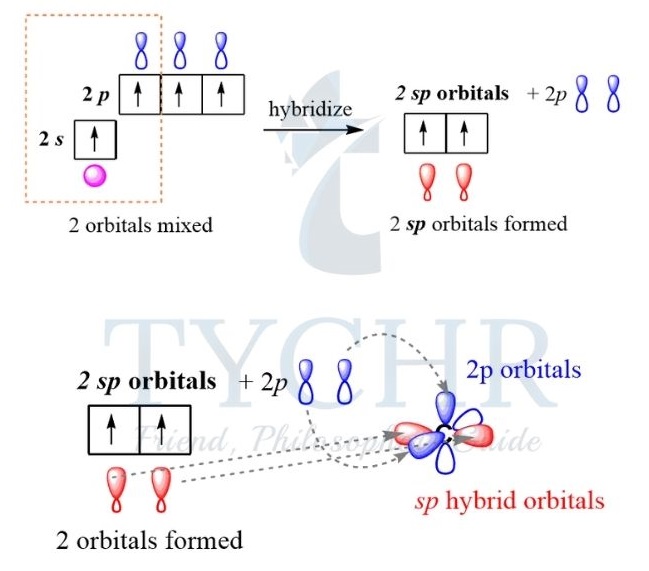
- When carbon forms a triple bond, it undergoes sp hybridization, producing two equal orbitals.
- These orbitals orientate themselves at 180°, giving a linear shape. Overlap of the two hybrid orbitals with other atomic-orbitals form two sigma bonds. For example, ethyne, C2H2.