periodicity Notes
Check Out Our Results

OXFORD UNIVERSITY
(QS:3)

IMPERIAL COLLEGE
(QS:6)

CORNELL UNIVERSITY (QS:16)

45/45 (IBDP)
GEORGIA INSTITUTE

43/45 (IBDP)
KELLY SCHOOL
The Periodic Table
Periods and groups
- In the modern periodic table the elements are arranged in order of increasing atomic number, Z, with elements having similar chemical and physical properties placed underneath each other in vertical columns called groups.
- The current periodic table consists of 118 elements.
- The horizontal rows of elements numbered from 1 to 7 are termed periods.
- The principal quantum number, n, of the highest occupied energy level in the period’s elements is the period number.
Group number
Recommended name
1
alkali metals
2
alkaline earth metals
15
pnictogens
16
chalcogens
17
halogens
18
noble gases
Metals, non-metals, and metalloids
- The periodic table is also split broadly into metals and non-metals; these are separated by a stepped diagonal line.
- The elements to the left of this line are the metals (excluding non-metallic hydrogen which is a gas) and the non-metals lie to the right.
Metal
Metals have the following properties:
- are excellent electrical and heat conductors
- are malleable (capable of being hammered into thin sheets)
- are ductile (capable of being drawn into wires)
- have lustre (they are shiny).
- Mercury, Hg, Z = 80, is a liquid and can dissolve many other metals.
- The solutions formed in this way are called amalgams. Non-metals:
- Heat and electricity are poor conductors of non-metals.
- Typically non-metals gain electrons in chemical reactions (they are reduced), whereas metals lose electrons (they are oxidized).
Metalloids
- Some of the elements close to the stepped diagonal line have both metallic and non-metallic properties.
- The elements boron, B, Z = 5, silicon, Si, Z = 14, germanium, Ge, Z = 32, arsenic, As, Z = 33, antimony, Sb, Z = 51, tellurium, Te, Z = 52, and astatine, At, Z = 85 are called the metalloids.
- Some metalloids such as silicon and germanium are semiconductors, due to their intermediate, highly temperature dependent electrical conductivity which has widespread applications in material science, such as in computers and smartphones.
Main group, transition elements, and s, p, d, and f blocks
- The periodic table can also be further divided into two broad sections.
- The main-group elements: group 1 (excluding H), group 2, and groups 13–18.
- The transition elements: groups 3–11.
- The properties of the main-group elements can often be predicted based on their position in the periodic table; this is less true for the properties of the transition elements.
- The periodic table is split into four blocks based on the s, p, d, and f sublevels.
Sublevels
Maximum number of electrons in sublevels
Number of atomic orbitals in each sublevel
s
2
1
p
6
3
d
10
5
f
14
7
- The number of valence electrons (outer-shell electrons) can be found from the group number of the s- and p-block elements,
- For example, calcium is in group 2, so has 2 valence electrons.
Main group elements
group 1 (excluding H), group 2, and groups 13–18
Transition elements
groups 3–11 (the f-block elements are sometimes described as the inner transition elements)
s-block elements
groups 1 and 2 and He
p- block elements
groups 13–18 (excluding He)
d- block elements
groups 3–12 (including Z = 57 (La) and Z = 89 (Ac), but excluding Z = 58 (Ce) to Z = 71 (Lu) and Z = 90 (Th) to Z = 103 (Lr), which are classified as f-block elements
f- block elements
elements from Z = 58 (Ce) to Z = 71 (Lu) and from Z = 90 (Th) to Z = 103 (Lr)
lanthanoids
elements from Z = 57 (La) to Z = 71 (Lu)
actinoids
elements from Z = 89 (Ac) to Z = 103 (Lr)
Periodic trends
Physical properties
Effective nuclear charge
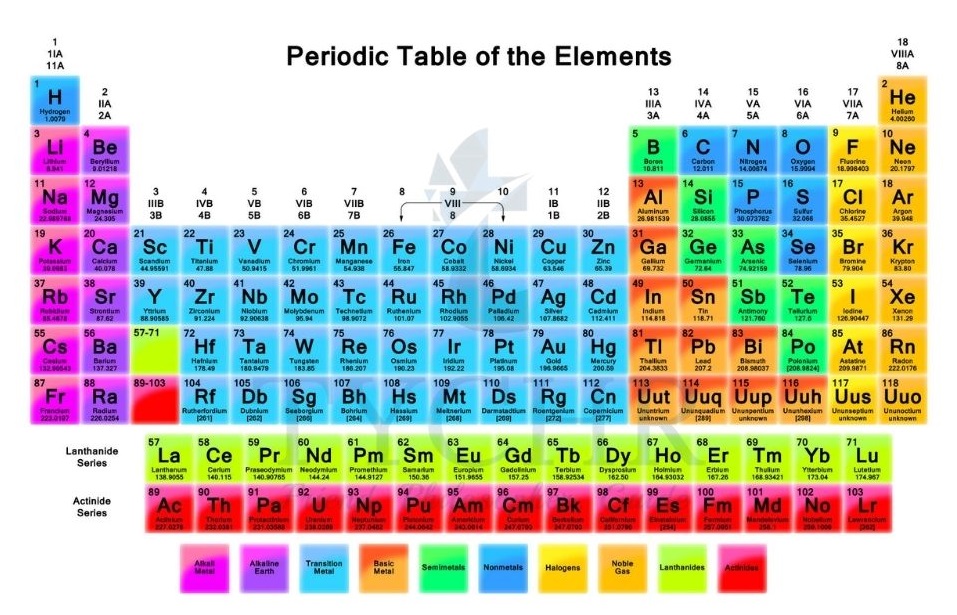
- The nuclear charge of the atom is given by the atomic number and so increases by one between successive elements in the table, as a proton is added to the nucleus.
- The outer electrons which determine many of the physical and chemical properties of the atom do not, however, experience the full attraction of this charge as they are shielded from the nucleus and repelled by the inner electrons.
- The presence of the inner electrons reduces the attraction of the nucleus for the outer electrons (Figure 1). The effective charge ‘experienced’ by the outer electrons is less than the full nuclear charge.
Figure 2: An electron in the hydrogen atom experiences the full attraction of the nuclear charge, but in a many electron atom the attraction for the nucleus is reduced as the outer electron is repelled by inner electrons.
Atomic radius
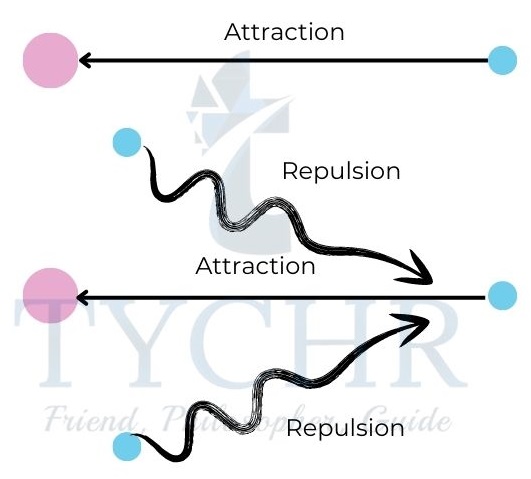
- The distance that separates a point on the circumference from the center of a circle is called its radius.
- In the Bohr model of the hydrogen atom the core of the atom is the nucleus while the single electron lies in a fixed orbit.
- The distance between the center of the nucleus and the outermost shell containing electrons is called the atomic radius.
- To put it another way, it is the distance that separates the electron cloud’s highest density point from the nucleus’s center.
- The atomic radius r is measured as half the distance between neighbouring nuclei.
Figure 2: An electron in the hydrogen
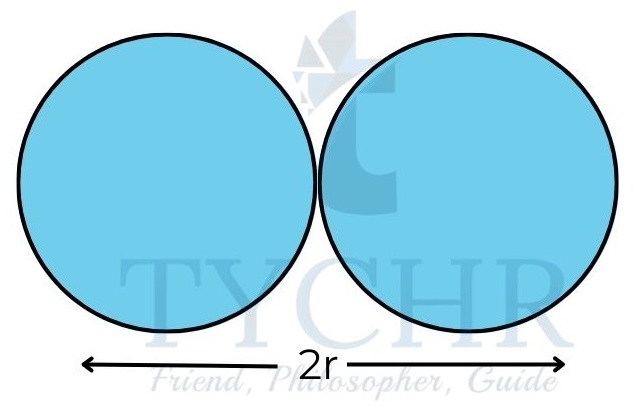
atom experiences the full attractionFigure 3: Trends in atomic radii. - Atomic radii generally decrease over time as a result of an increase in the attraction that exists between the nucleus and the outer electrons.
- The decrease in radii across a period is quite significant; a chlorine atom, for example, has a radius that is about half that of a sodium atom.
Ionic radius
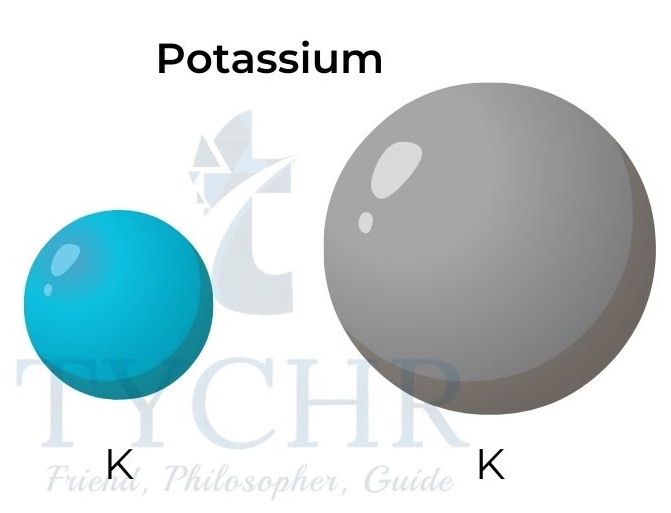
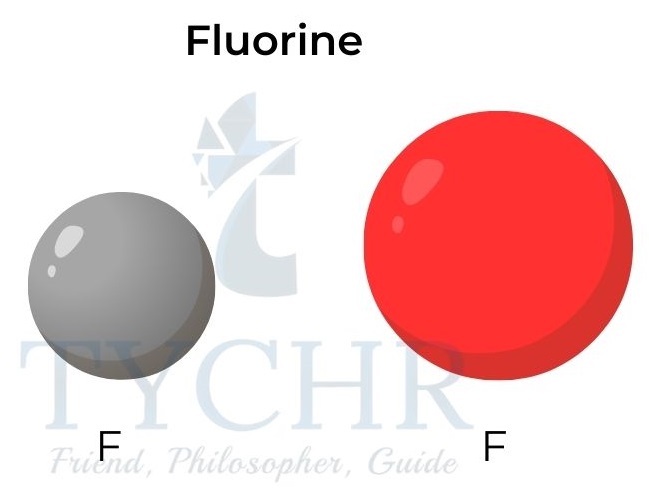
- Positive ions are smaller than their parent atoms. The formation of positive ions involves the loss of the outer shell.
- Negative ions are larger than their parent atoms. The formation of negative ions involves the addition of electrons into the outer shell.
- The ionic radii decrease from Groups 1 to 14 for the positive ions. The ions Na+, Mg2+, Al3+, and Si4+ all have the same electron configuration (1s22s22p6).
- The ionic radii decrease from Groups 14 to 17 for the negative ions. The ions Si4–, P3–, S2– and Cl– have the same electron configuration (1s22s22p63s23p6).
- The ionic radii increase down a group as the number of electron energy levels increases.
Figure 4: Atomic and ionic radii for potassium and fluorine.
Ionization energy
- The ionization energy, IE, is the minimum energy required to remove an electron from a neutral gaseous atom in its ground-state.
- First ionization energies are a measure of the attraction between the nucleus and the outer electrons.
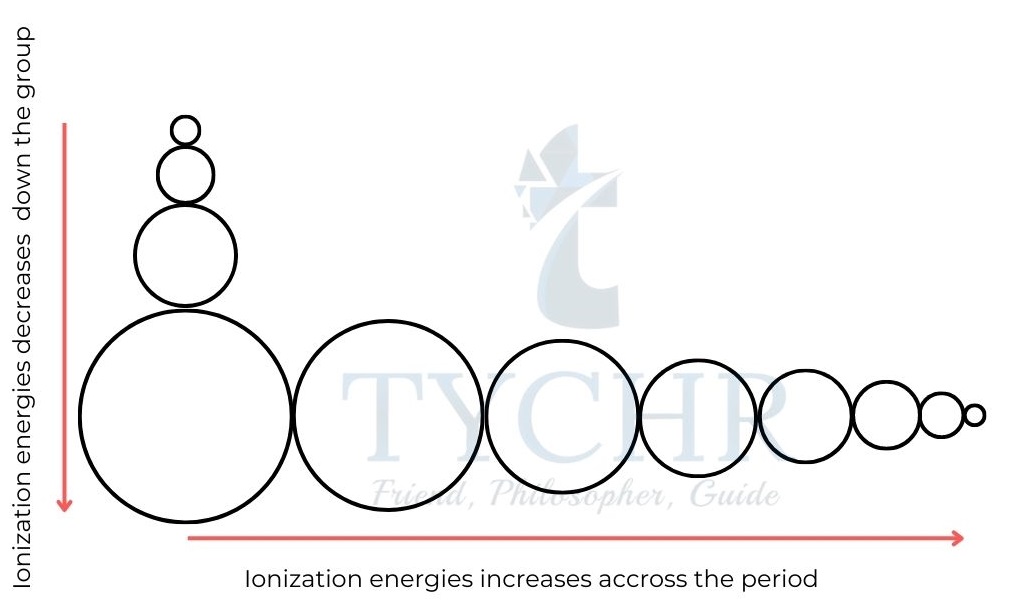
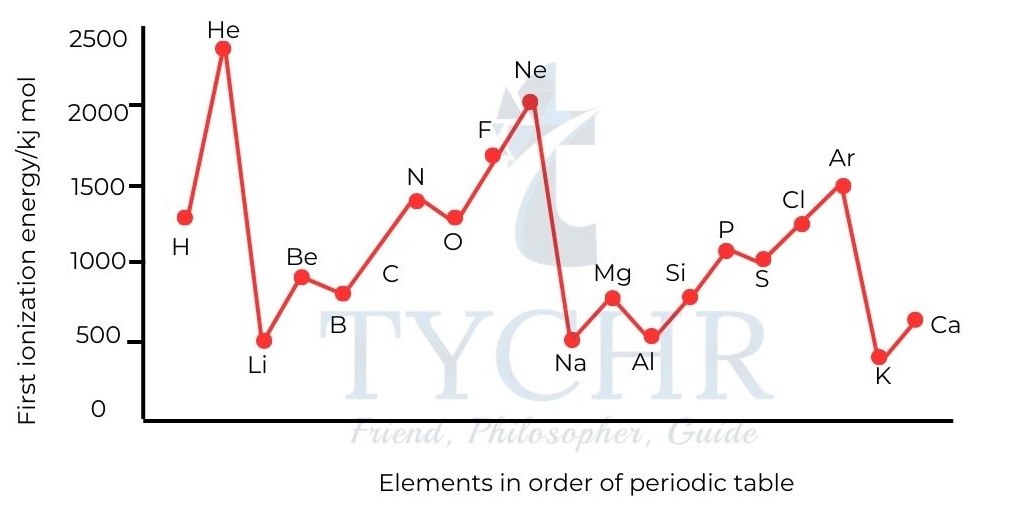
Figure 5: First ionization energies of the first 20 elements. - Ionization energies increase across a period. The attraction between the outer electrons and the nucleus increases as effective nuclear charge rises, making it more challenging to remove the electrons. Ionization energies decrease down a group. The electron removed is from the energy level furthest from the nucleus.
- Although the nuclear charges increase, the effective nuclear charge is about the same, owing to shielding of the inner electrons, and so the increased distance between the electron and the nucleus reduces the attraction between them.
- Atomic radii are moving in the opposite direction of the trend in ionization energy. The attraction between the nucleus and the outer electrons can be seen in these two trends.
Figure 6: Trends in ionization energy are the opposite of the trends in atomic radius.
Electron affinity
- The first electron affinity of an element (∆Hea) is the energy change when one mole of electrons is added to one mole of gaseous atoms to form one mole of gaseous ions:
X(g) + e– → X–(g) - The noble gases do not generally form negatively charged ions so electron affinity values are not available for these elements.
- The added electron is attracted to the positively charged nucleus and the process is generally exothermic.
- The second electron affinity for oxygen, for example, corresponds to the change:
O–(g) + e– → O2–(g) - This process is endothermic as the added electron is repelled by the negatively charged oxide (O–) ion, and energy needs to be available for this to occur.
- Electron affinities can be thought of as the negative of the first ionization energy of the anion.
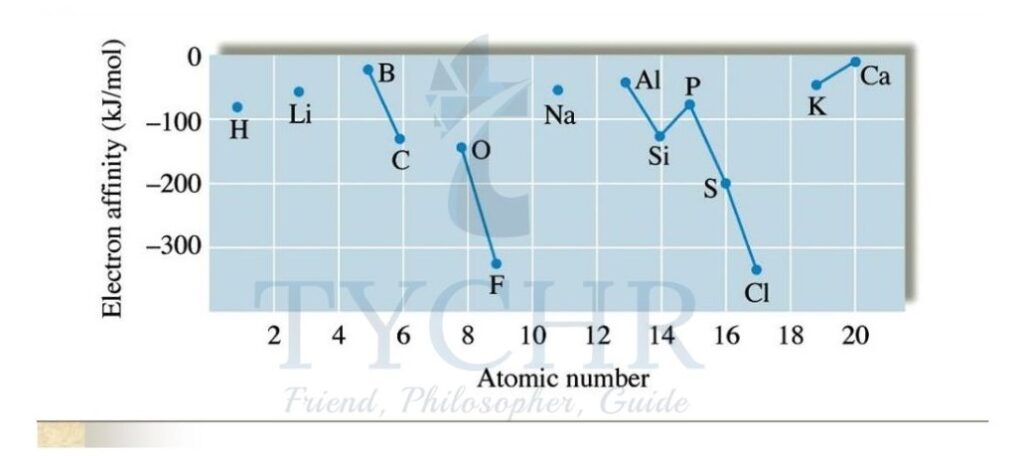
- The Group 17 elements have incomplete outer energy levels and a high effective nuclear charge of approximately +7 and so attract electrons the most.
- The Group 1 metals have the lowest effective nuclear charge of approximately +1 and so attract the extra electron the least.
Figure 7: The electron affinities of the first 18 elements. Note there are no values assigned to the noble gases.
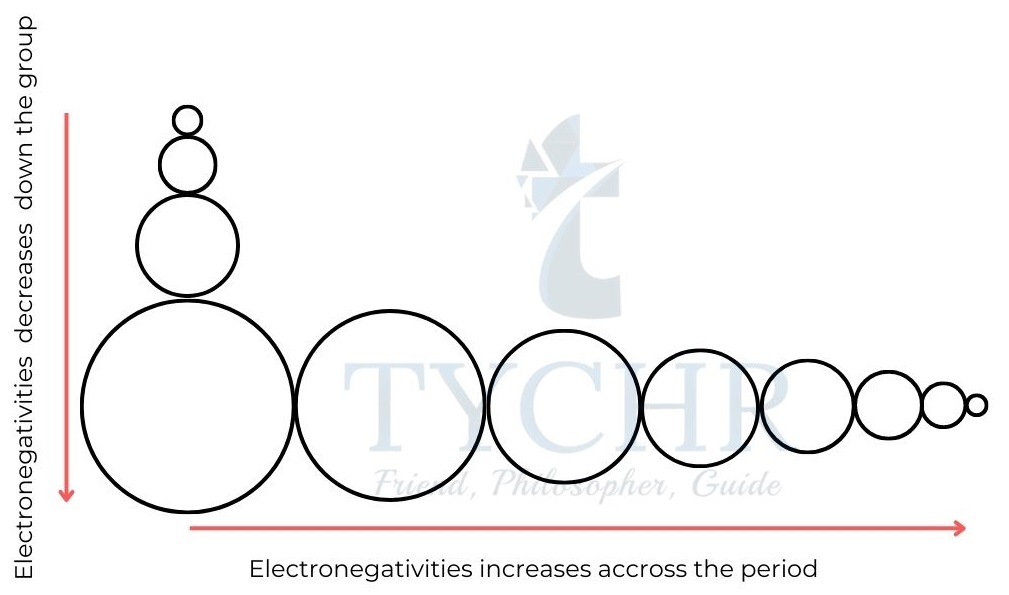
Electronegativity
- Electronegativity, symbol χ, is defined as the relative attraction that an atom has for the shared pair of electrons in a covalent bond.
- The effective nuclear charge and atomic radii both increase over time, resulting in an increase in electronegativity values from left to right.
- Down a group from top to bottom electronegativity values decrease because atomic radii increase and although the nuclear charge, Z, increases, its effect is shielded by the core electrons.
Figure 2: An electron in the hydrogen atom experiences the full attraction of the Figure 8: Trends in electronegativity are the same as those in ionizanuclear charge, but in a many electron atom the attraction for the nucleus is reduced as the outer electron is repelled by inner electrons.
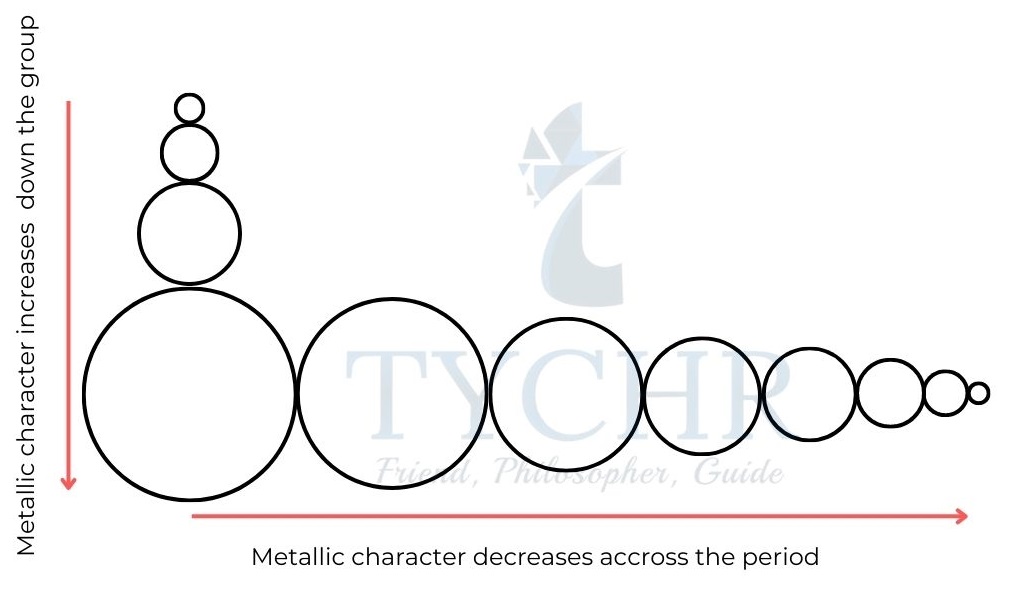
Metallic and non-metallic character
- Metallic character increases as one moves down a group and decreases across time.
- Metals also have low ionization energy values – they have a tendency to lose electrons during a chemical reaction, that is, they tend to be oxidized.
- Non-metals show highly negative electron affinities – they have a tendency to gain electrons during a chemical reaction, that is, they tend to be reduced.
Figure 9: Trends in metallic character in the periodic table.
Chemical properties
Group 18: the noble gases
- They are colourless gases.
- They are monatomic: they exist as single atoms.
- They are very unreactive.
- Their lack of reactivity can be explained by the inability of their atoms to lose or gain electrons.
- Elements in Groups 1, 2, and 13 lose electrons to adopt the arrangement of the nearest noble gas with a lower atomic number. They are generally metals.
- Elements in Groups 15 to 17 gain electrons to adopt the electron configuration of the nearest noble gas on their right in the Periodic Table. They are generally non-metals.
- The metalloids in the middle of the table show intermediate properties.
Group 1: the alkali metals
- Silvery metals make up all of the elements, which are too reactive to be found in nature.
- They are usually stored in oil to prevent contact with air and water.
Physical properties
Chemical properties
They are good conductors of electricity and heat.
They are very reactive metals.
They have low densities.
They form ionic compounds with non-metals.
They have grey shiny surfaces when freshly cut with a knife.
Group 17: the halogens
- The Group 17 elements exist as diatomic molecules, X2.
Physical properties
Chemical properties
They are coloured.
They are very reactive non-metals. Reactivity decreases down the group
They show a gradual change from gases (F2 and Cl2), to liquid (Br2), and solids (I2 and At2).
They form ionic compounds with metals and covalent compounds with other non-metals.
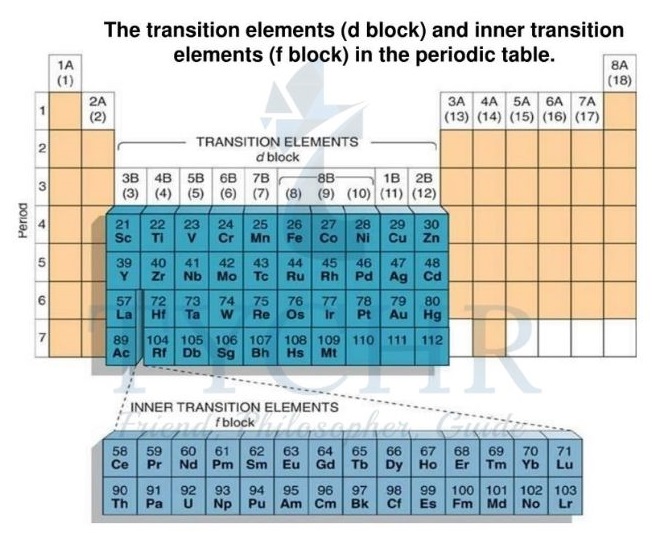
First-row d-block elements
Transition elements
- At the centre of the periodic table of elements lies a very important family of elements, called the transition elements, whose physical and chemical properties often play a key role in many facets of everyday life.
- A transition element is an element that has an atom with an incomplete d-sublevel or that gives rise to cations with an incomplete d-sublevel.
- In the periodic table (figure 1) the first-row transition elements are the elements in period 4 from scandium (Sc) to copper (Cu) inclusive. The elements below these elements in periods 5, 6, and 7 are also described as transition elements.
- The f-block elements are sometimes described as the inner transition elements.
- Collectively, the elements in groups 3–12 inclusive (including La and Ac) are referred to as the
d-block elements. - The elements that fill the 4f and 5f orbitals are the ones that make up the f-block.
- These elements are formal members of group 3 but they form a separate f-block in the periodic table (figure 2).
- The metallic nature of the transition elements means they are often described as the transition metals.
Figure 10: Different block elements in periodic table.
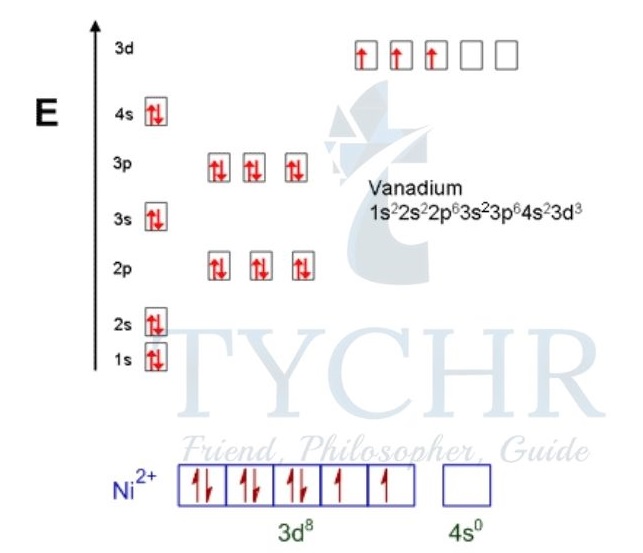
Electron configurations of first-row d-block elements and their ions
- The following are some examples of full and condensed electron configurations of the first- row d-block elements, their ions, and their corresponding orbital diagrams:
- For vanadium, V (Z = 23):
1s22s22p63s23p63d34s2 (full electron configuration) [Ar]3d34s2 (condensed electron configuration) The orbital diagram is: - For Ni2+ (Z = 28): 1s22s22p63s23p63d8 (full electron configuration) [Ar]3d8 (condensed electron configuration) The orbital diagram is:
In the case of Ni2+ the electrons are removed from the 4s level before the 3d level.
Characteristics of transition elements
- Transition metals have a number of key characteristics:
- They have variable oxidation states
- Compounds of transition elements and their ions are often coloured
- Transition metals form complexes with ligands
- As catalysts, transition metals are frequently utilized.
- Magnetic properties of transition metals depend on their oxidation states and coordination number.
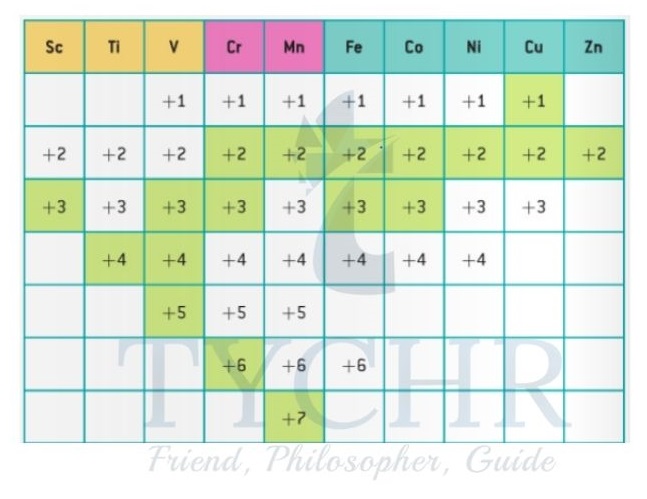
Variable oxidation states
- In contrast to an alkali metal such as sodium, where the oxidation state is always +1 in its ions and compounds, transition metals are often found with different oxidation states.
- The range of different oxidation states for the first-row d-block elements can be seen from the diagram shown in figure 11. The d-block elements can be split according to their oxidation states into three types – A, B, and C.
- The characteristics of Type A are dominated by:
- Stable, high oxidation states (for example, V is +5 in VO – )
Unstable low oxidation states.
The characteristics of Type B are dominated by:
- Stable high oxidation states (for example, Mn is +7 in MnO4 , Cr is +6 in Cr2O72- )
- Stable low oxidation states (for example, Mn is +2 in [Mn (H2O)6]2+, Cr is +3 in [Cr(H2O)6]3+).
The characteristics of Type C are dominated by:
- Unstable high oxidation states
- Stable low oxidation states (for example, Fe is +2 in [Fe(H2O)6]2+).
Figure 11: Range of oxidation states of the first-row d-block metals. The most common oxidation states are marked in green.
Figure 11: Range of oxidation states of the first-row d-block metals. The most common oxidation states are marked in green.
Coloured compounds of transition metals and their ions
- Transition metal compounds and ions are often coloured, for example:
- KMnO4: burgundy (purple), [Mn(H2O)6]2+ :almost colourless, with a faint pink tinge, K2Cr2O7
:orange, [Cr(H2O)6]3+: green, CuSO4 5H2O: blue, [NH4]2[Fe(H2O)6][SO4]2: pale green. - Compounds that contain transition elements and in which the central metal ion, Mn+, is bonded, via coordinate bonding, to a group of molecules or ions (termed the ligands) are termed transition metal complexes.
- Such compounds are often described as coordination compounds, to signify the coordinate bonding present between the ligand(s) and the central metal ion.
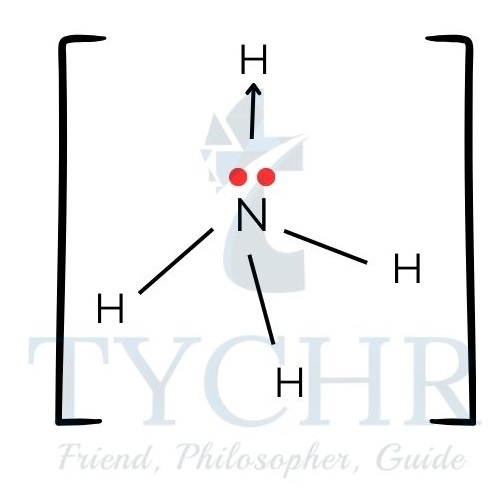
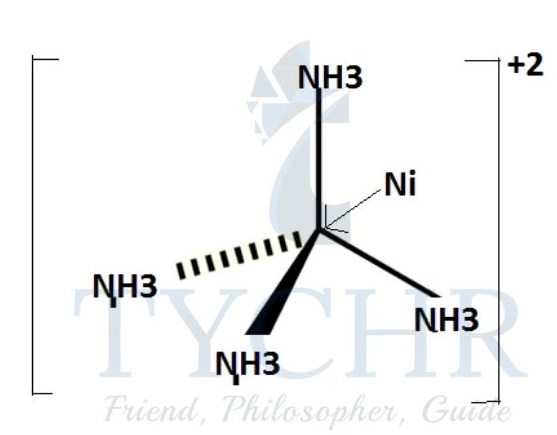
- Examples of species with coordinate bonding:
hydronium cation, [H3O]+:
carbon monoxide, CO: ammonium cation, [NH4]+:
a transition metal complex, for example [Ni(NH3)6]2+:
Classification of ligands
- The number of coordinate bonds formed by one ligand with a metal ion depends on the number of donor centres (atoms with lone electron pairs) in the ligand.
- Monodentate ligands are able to form only one coordinate bond with a metal ion while polydentate ligands (also known as chelate ligands) can form two or more such bonds.
- Monodentate ligands contain a single donor atom and have one lone pair contributing to the coordinate bond in a complex. Typical examples include water, ammonia, and the halides such as Cl– etc.
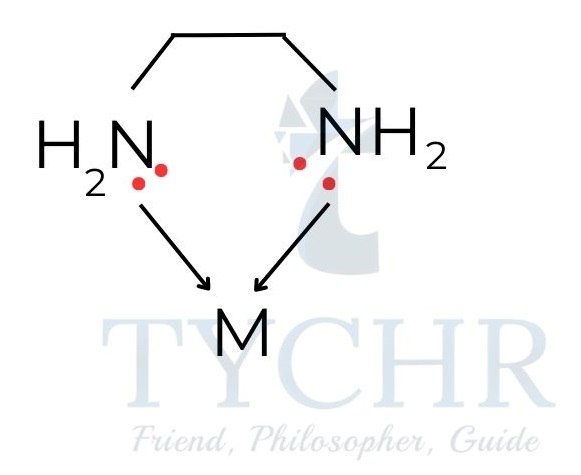
- Polydentate (chelate) ligands are ligands which have two or more donor atoms that form coordinate bonds with a transition metal centre.
- Example : 1,2-ethanediamine (en), H2NCH2CH2NH2
Transition metals as catalysts
- In chemical reactions, transition metals are frequently utilized as catalysts. Some examples of reactions are as follows:
- Haber process: N2(g) + 3H2(g) ⇋ 2NH3(g) ; catalyst: Fe(s)
- Hydrogenation of alkenes: H2C=CH2(g) + H2(g) → CH3CH3(g) ; catalyst: Ni(s), Pd(s), or Pt(s)
- Decomposition of hydrogen peroxide: 2H2O2(aq) → 2H2O(l) + O2(g); catalyst: MnO2(s)
- Hydrogenation of oils: RCH=CHR’ + H2(g) → RCH2CH2R’; catalyst: Ni(s)
Magnetic properties of transition metals
- Magnetic properties of transition metals and their complexes depend on many factors, including the oxidation state of the metal, its coordination number, and the geometry of the complex.
- Paramagnetic materials contain unpaired electrons that behave as tiny magnets and are attracted by an external magnetic field.
- Diamagnetic materials, on the other hand, lack unpaired electrons and are thus repellent to external magnetic fields.
Coloured complexes
The visible spectrum
- The visible spectrum ranges from 400 nm to about 700 nm. The colour we see depends on wavelength.
Colour
Wavelength range / nm
Red
630–700
Orange
590–630
Yellow
560–590
Green
490–560
Blue
450–490
Violet
400–450
Table 6: The colour we see depends on wavelength.
- The colour of a substance is determined by which colour(s) of light it absorbs and which colour(s) it transmits or reflects (the complementary colour(s)).
Transition metals appear coloured because they absorb visible light
- White light is composed of all the colours of the visible spectrum.
- Transition metal compounds appear coloured because their ions absorb some of these colours.
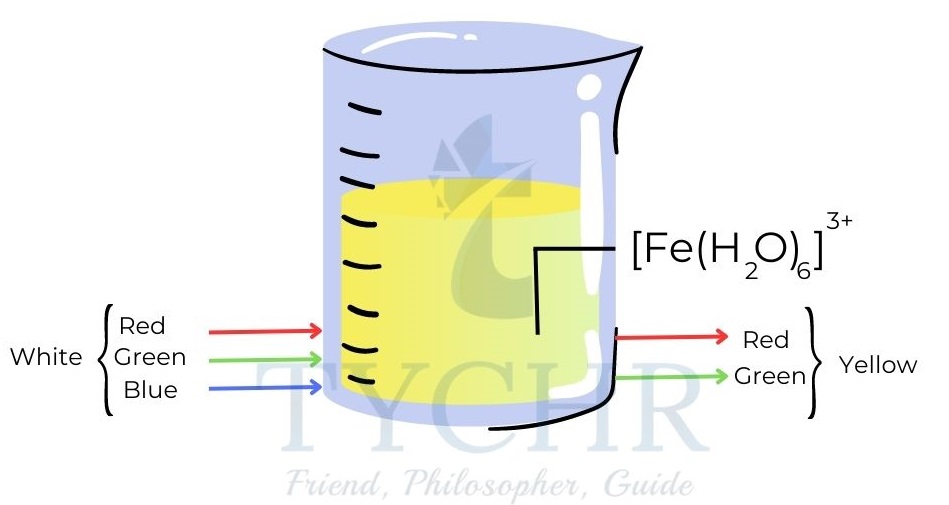
- [Fe (H2O)6]3+, for example, appears yellow because it absorbs light in the blue region of the spectrum.
Figure 12: The ion Fe3+ appears yellow because it absorbs blue light. Yellow is the complementary colour to blue.
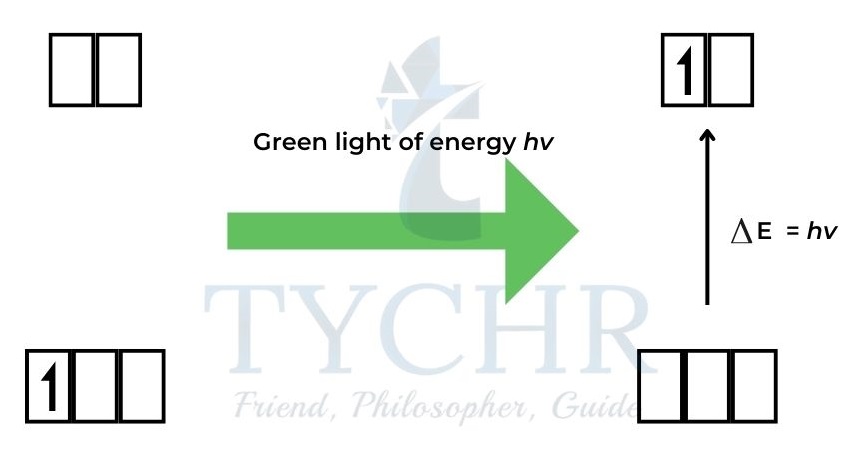
Transition metals absorb light because the d orbitals split into two sub-levels
- The d orbitals in an isolated transition metal atom are said to be degenerate as they all have the same energy.
- The energy separation between the orbitals is ∆E and hence the colour of the complex
depends on the following factors: - The nuclear charge and the identity of the central metal ion;
- The charge density of the ligand;
- The geometry of the complex ion (the electric field created by the ligand’s lone pair of electrons depends on the geometry of the complex ion);
- The number of d electrons present and hence the oxidation number of the central ion.
Figure 13: Green light of energy hν excites an electron from a d orbital of lower energy to a d orbital of higher energy. The transmitted light is purple.